Antiviral efficacy against and replicative fitness of an XBB.1.9.1 clinical isolate
et al., iScience, doi:10.1016/j.isci.2023.108147, Nov 2023
42nd treatment shown to reduce risk in
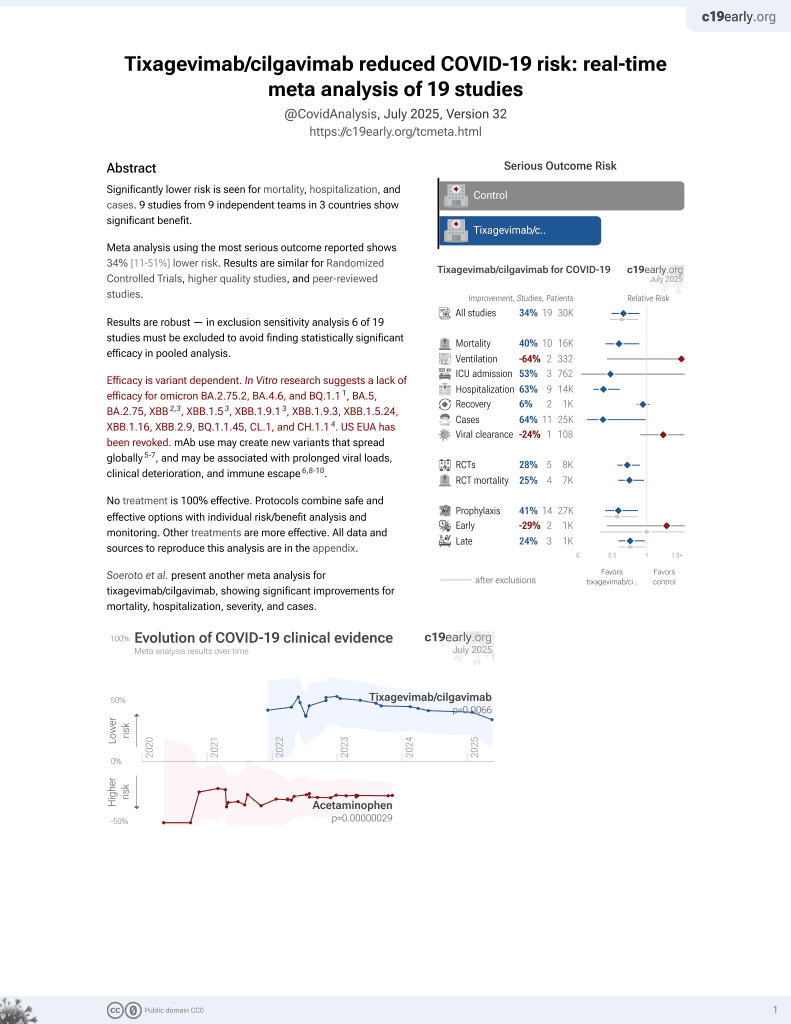
May 2022, now with p = 0.0066 from 19 studies, recognized in 33 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
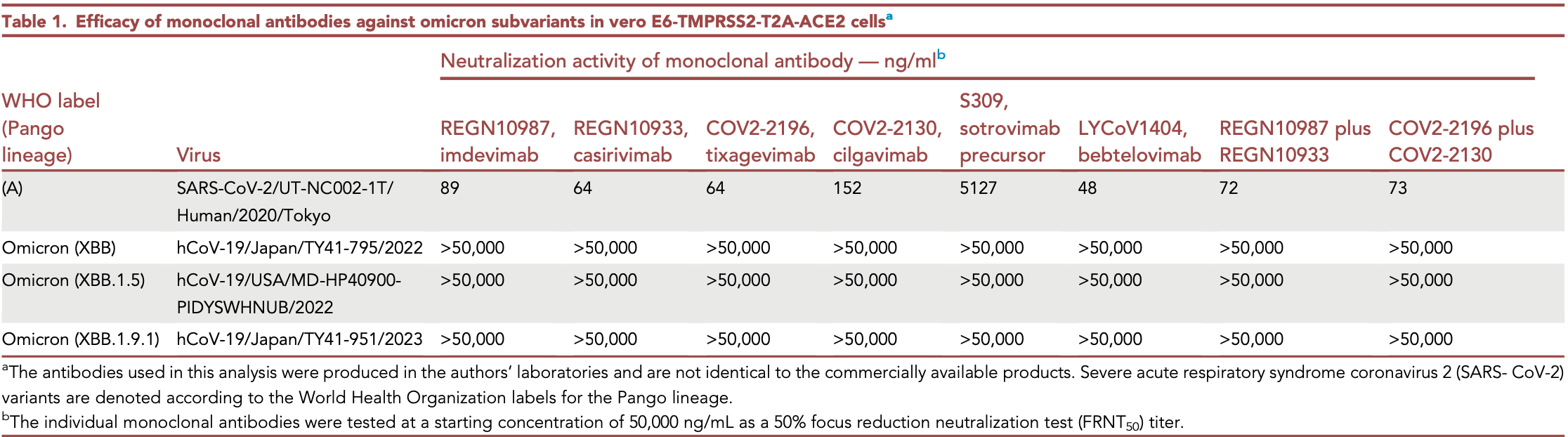
In vitro and animal study showing that the SARS-CoV-2 omicron subvariant XBB.1.9.1 has similar antigenicity, antiviral susceptibility, and replicative ability compared to XBB.1.5. Casirivimab, imdevimab, tixagevimab, cilgavimab, sotrovimab, and bebtelovimab were not effective against XBB, XBB.1.5, and XBB.1.9.1. XBB.1.9.1 and XBB.1.5 remained susceptible to remdesivir, molnupiravir, nirmatrelvir, and ensitrelvir. In hamsters infected with a mixture of the two variants, XBB.1.9.1 and XBB.1.5 showed similar replicative fitness in nasal turbinates and lungs.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2.75.2, BA.4.6, BQ.1.11, BA.5, BA.2.75, XBB2,3, XBB.1.53, ХВВ.1.9.13, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.14.
Study covers casirivimab/imdevimab, tixagevimab/cilgavimab, sotrovimab, bebtelovimab, ensitrelvir, paxlovid, molnupiravir, and remdesivir.
1.
Planas et al., Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies, bioRxiv, doi:10.1101/2022.11.17.516888.
2.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Uraki et al., 30 Nov 2023, peer-reviewed, 20 authors.
Contact: yoshihiro.kawaoka@wisc.edu (corresponding author), yoshihiro.kawaoka@wisc.edu (corresponding author).
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Antiviral efficacy against and replicative fitness of an XBB.1.9.1 clinical isolate
iScience, doi:10.1016/j.isci.2023.108147
Highlights The antigenicity of XBB.1.9.1 is similar to that of XBB.1.5 XBB.1.9.1 remains susceptible to antiviral drugs The replicative ability of XBB.1.9.1 is comparable to that of XBB.1.5
SUPPLEMENTAL INFORMATION Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.108147 .
AUTHOR CONTRIBUTIONS R.U.: conceptualization, formal analysis, validation, visualization, and writing of the first draft. M. Ito, M. Kiso: data curation, formal analysis, and methodology. S. Yamayoshi: conceptualization, data curation, formal analysis, and methodology. K.I-H.: resources and validation. Y.S-T., M.
Data and code availability All data used in this paper are available in the main text, in the supplemental information, or the sources have been clearly stated. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND STUDY PARTICIPANT DETAILS Animal studies were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocols were approved by the Animal Experiment Committee of the Institute of Medical Science, the University of Tokyo (approval number PA19-75). Virus inoculations were performed under isoflurane, and all efforts were made to minimize animal suffering. To collect and use clinical specimens, the research protocol was approved by the Research Ethics Review Committee of the Institute of Medical Science of the University of Tokyo (approval numbers: 2019-71-0201 and 2020-740226). After informed consent was..
References
Chen, Li, Peng, Tian, Ji et al., Neutralization against XBB.1 and XBB.1.5 after omicron subvariants breakthrough infection or reinfection, Lancet Reg. Health. West. Pac, doi:10.1016/j.lanwpc.2023.100759
Imai, Halfmann, Yamayoshi, Iwatsuki-Horimoto, Chiba et al., Characterization of a new SARS-CoV-2 variant that emerged in Brazil, Proc. Natl. Acad. Sci, doi:10.1073/pnas.2106535118
Imai, Ito, Kiso, Yamayoshi, Uraki et al., Efficacy of Antiviral Agents against Omicron Subvariants BQ.1.1 and XBB, N. Engl. J. Med, doi:10.1056/NEJMc2214302
Imai, None
Imai, were propagated in the presence of 1 mg/ml geneticin (G418; Invivogen) and 5 mg/ml plasmocin prophylactic (Invivogen) in DMEM containing 10% FCS. Vero E6-TMPRSS2-T2A-ACE2 and VeroE6/TMPRSS2 cells were maintained at 37 C with 5% CO 2 . Chinese hamster ovary (CHO) cells were maintained in DMEM containing 10% FCS and antibiotics at 37 C with 5% CO 2 . Expi293F cells
Matsuyama, Nao, Shirato, Kawase, Saito et al., Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells, Proc. Natl. Acad. Sci, doi:10.1073/pnas.2002589117
Matsuyama, None
Matsuyama, None
Matsuyama, None
Muramoto, Takahashi, Halfmann, Gotoh, Noda et al., Replicative capacity of SARS-CoV-2 omicron variants BA.5 and BQ.1.1 at elevated temperatures, Lancet. Microbe, doi:10.1016/S2666-5247(23)00100-3
Qu, Faraone, Evans, Zheng, Carlin et al., Enhanced evasion of neutralizing antibody response by Omicron XBB.1.5, CH.1.1, and CA.3.1 variants, Cell Rep, doi:10.1016/j.celrep.2023.112443
Takashita, Kinoshita, Yamayoshi, Sakai-Tagawa, Fujisaki et al., Efficacy of Antibodies and Antiviral Drugs against Covid-19 Omicron Variant, N. Engl. J. Med, doi:10.1056/NEJMc2119407
Takashita, Morita, Ogawa, Nakamura, Fujisaki et al., Susceptibility of Influenza Viruses to the Novel Cap-Dependent Endonuclease Inhibitor Baloxavir Marboxil, Front. Microbiol, doi:10.3389/fmicb.2018.03026
Takashita, Yamayoshi, Simon, Van Bakel, Sordillo et al., Efficacy of Antibodies and Antiviral Drugs against Omicron BA.2.12.1, BA.4, BA.5 Subvariants. N. Engl. J. Med, doi:10.1056/NEJMc2207519
Uraki, Ito, Kiso, Yamayoshi, Iwatsuki-Horimoto et al., Antiviral and bivalent vaccine efficacy against an omicron XBB.1.5 isolate, Lancet Infect. Dis, doi:10.1016/S1473-3099(23)00070-1
Uraki, Ito, Kiso, Yamayoshi, Iwatsuki-Horimoto et al., Efficacy of antivirals and mRNA vaccination against an XBF clinical isolate, Lancet Reg. Health. West. Pac, doi:10.1016/j.lanwpc.2023.100777
Uriu, Ito, Zahradnik, Fujita, Kosugi et al., Enhanced transmissibility, infectivity, and immune resistance of the SARS-CoV-2 omicron XBB.1.5 variant, Lancet Infect. Dis, doi:10.1016/S1473-3099(23)00051-8
Vanderheiden, Edara, Floyd, Kauffman, Mantus et al., Development of a Rapid Focus Reduction Neutralization Test Assay for Measuring SARS-CoV-2 Neutralizing Antibodies, Curr. Protoc. Immunol, doi:10.1002/cpim.116
Yue, Song, Wang, Jian, Chen et al., ACE2 binding and antibody evasion in enhanced transmissibility of XBB.1.5, Lancet Infect. Dis, doi:10.1016/S1473-3099(23)00010-5
DOI record:
{
"DOI": "10.1016/j.isci.2023.108147",
"ISSN": [
"2589-0042"
],
"URL": "http://dx.doi.org/10.1016/j.isci.2023.108147",
"alternative-id": [
"S2589004223022241"
],
"article-number": "108147",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Antiviral efficacy against and replicative fitness of an XBB.1.9.1 clinical isolate"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "iScience"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.isci.2023.108147"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 The Author(s)."
}
],
"author": [
{
"affiliation": [],
"family": "Uraki",
"given": "Ryuta",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ito",
"given": "Mutsumi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kiso",
"given": "Maki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yamayoshi",
"given": "Seiya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Iwatsuki-Horimoto",
"given": "Kiyoko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sakai-Tagawa",
"given": "Yuko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Imai",
"given": "Masaki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Koga",
"given": "Michiko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yamamoto",
"given": "Shinya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Adachi",
"given": "Eisuke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saito",
"given": "Makoto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tsutsumi",
"given": "Takeya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Otani",
"given": "Amato",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fukushi",
"given": "Shuetsu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Watanabe",
"given": "Shinji",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Suzuki",
"given": "Tadaki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kikuchi",
"given": "Tetsuhiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yotsuyanagi",
"given": "Hiroshi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maeda",
"given": "Ken",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5061-8296",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kawaoka",
"given": "Yoshihiro",
"sequence": "additional"
}
],
"container-title": "iScience",
"container-title-short": "iScience",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"cell.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
10,
4
]
],
"date-time": "2023-10-04T08:42:34Z",
"timestamp": 1696408954000
},
"deposited": {
"date-parts": [
[
2024,
8,
5
]
],
"date-time": "2024-08-05T06:56:12Z",
"timestamp": 1722840972000
},
"indexed": {
"date-parts": [
[
2024,
10,
6
]
],
"date-time": "2024-10-06T01:16:09Z",
"timestamp": 1728177369351
},
"is-referenced-by-count": 5,
"issue": "11",
"issued": {
"date-parts": [
[
2023,
11
]
]
},
"journal-issue": {
"issue": "11",
"published-print": {
"date-parts": [
[
2023,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
1
]
],
"date-time": "2023-11-01T00:00:00Z",
"timestamp": 1698796800000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
1
]
],
"date-time": "2023-11-01T00:00:00Z",
"timestamp": 1698796800000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
10,
2
]
],
"date-time": "2023-10-02T00:00:00Z",
"timestamp": 1696204800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2589004223022241?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2589004223022241?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "108147",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
11
]
]
},
"published-print": {
"date-parts": [
[
2023,
11
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"article-title": "Neutralization against XBB.1 and XBB.1.5 after omicron subvariants breakthrough infection or reinfection",
"author": "Chen",
"first-page": "100759",
"journal-title": "Lancet Reg. Health. West. Pac.",
"key": "10.1016/j.isci.2023.108147_bib1",
"volume": "33",
"year": "2023"
},
{
"DOI": "10.1016/j.celrep.2023.112443",
"article-title": "Enhanced evasion of neutralizing antibody response by Omicron XBB.1.5, CH.1.1, and CA.3.1 variants",
"author": "Qu",
"doi-asserted-by": "crossref",
"first-page": "112443",
"journal-title": "Cell Rep.",
"key": "10.1016/j.isci.2023.108147_bib2",
"volume": "42",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(23)00070-1",
"article-title": "Antiviral and bivalent vaccine efficacy against an omicron XBB.1.5 isolate",
"author": "Uraki",
"doi-asserted-by": "crossref",
"first-page": "402",
"journal-title": "Lancet Infect. Dis.",
"key": "10.1016/j.isci.2023.108147_bib3",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(23)00051-8",
"article-title": "Enhanced transmissibility, infectivity, and immune resistance of the SARS-CoV-2 omicron XBB.1.5 variant",
"author": "Uriu",
"doi-asserted-by": "crossref",
"first-page": "280",
"journal-title": "Lancet Infect. Dis.",
"key": "10.1016/j.isci.2023.108147_bib4",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(23)00010-5",
"article-title": "ACE2 binding and antibody evasion in enhanced transmissibility of XBB.1.5",
"author": "Yue",
"doi-asserted-by": "crossref",
"first-page": "278",
"journal-title": "Lancet Infect. Dis.",
"key": "10.1016/j.isci.2023.108147_bib5",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1016/S2666-5247(23)00100-3",
"article-title": "Replicative capacity of SARS-CoV-2 omicron variants BA.5 and BQ.1.1 at elevated temperatures",
"author": "Muramoto",
"doi-asserted-by": "crossref",
"first-page": "e486",
"journal-title": "Lancet. Microbe",
"key": "10.1016/j.isci.2023.108147_bib6",
"volume": "4",
"year": "2023"
},
{
"DOI": "10.1056/NEJMc2119407",
"article-title": "Efficacy of Antibodies and Antiviral Drugs against Covid-19 Omicron Variant",
"author": "Takashita",
"doi-asserted-by": "crossref",
"first-page": "995",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.isci.2023.108147_bib7",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2207519",
"article-title": "Efficacy of Antibodies and Antiviral Drugs against Omicron BA.2.12.1, BA.4, and BA.5 Subvariants",
"author": "Takashita",
"doi-asserted-by": "crossref",
"first-page": "468",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.isci.2023.108147_bib8",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2214302",
"article-title": "Efficacy of Antiviral Agents against Omicron Subvariants BQ.1.1 and XBB",
"author": "Imai",
"doi-asserted-by": "crossref",
"first-page": "89",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.isci.2023.108147_bib9",
"volume": "388",
"year": "2023"
},
{
"article-title": "Efficacy of antivirals and mRNA vaccination against an XBF clinical isolate",
"author": "Uraki",
"first-page": "100777",
"journal-title": "Lancet Reg. Health. West. Pac.",
"key": "10.1016/j.isci.2023.108147_bib10",
"volume": "34",
"year": "2023"
},
{
"DOI": "10.1073/pnas.2002589117",
"article-title": "Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells",
"author": "Matsuyama",
"doi-asserted-by": "crossref",
"first-page": "7001",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "10.1016/j.isci.2023.108147_bib11",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2106535118",
"article-title": "Characterization of a new SARS-CoV-2 variant that emerged in Brazil",
"author": "Imai",
"doi-asserted-by": "crossref",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "10.1016/j.isci.2023.108147_bib12",
"volume": "118",
"year": "2021"
},
{
"DOI": "10.1002/cpim.116",
"article-title": "Development of a Rapid Focus Reduction Neutralization Test Assay for Measuring SARS-CoV-2 Neutralizing Antibodies",
"author": "Vanderheiden",
"doi-asserted-by": "crossref",
"first-page": "e116",
"journal-title": "Curr. Protoc. Immunol.",
"key": "10.1016/j.isci.2023.108147_bib13",
"volume": "131",
"year": "2020"
},
{
"DOI": "10.3389/fmicb.2018.03026",
"article-title": "Susceptibility of Influenza Viruses to the Novel Cap-Dependent Endonuclease Inhibitor Baloxavir Marboxil",
"author": "Takashita",
"doi-asserted-by": "crossref",
"first-page": "3026",
"journal-title": "Front. Microbiol.",
"key": "10.1016/j.isci.2023.108147_bib14",
"volume": "9",
"year": "2018"
}
],
"reference-count": 14,
"references-count": 14,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2589004223022241"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Antiviral efficacy against and replicative fitness of an XBB.1.9.1 clinical isolate",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "26"
}
uraki