Intramuscular AZD7442 (Tixagevimab–Cilgavimab) for Prevention of Covid-19
et al., New England Journal of Medicine, doi:10.1056/NEJMoa2116620, PROVENT, NCT04625725, Apr 2022
42nd treatment shown to reduce risk in
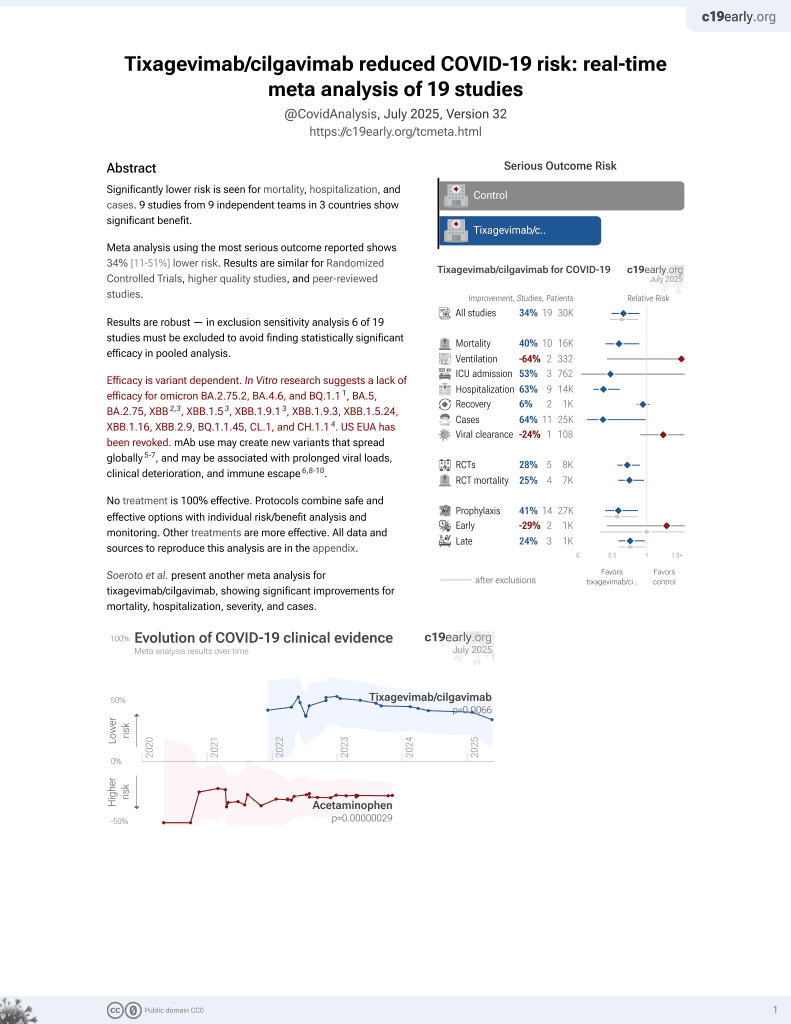
May 2022, now with p = 0.0066 from 19 studies, recognized in 33 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
PrEP RCT with 3,441 tixagevimab/cilgavimab patients and 1,731 control patients, showing lower risk of symptomatic cases with treatment.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2.75.2, BA.4.6, BQ.1.11, BA.5, BA.2.75, XBB2,3, XBB.1.53, ХВВ.1.9.13, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.14.
|
risk of death, 85.7% lower, RR 0.14, p = 0.11, treatment 0 of 3,441 (0.0%), control 2 of 1,731 (0.1%), NNT 866, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
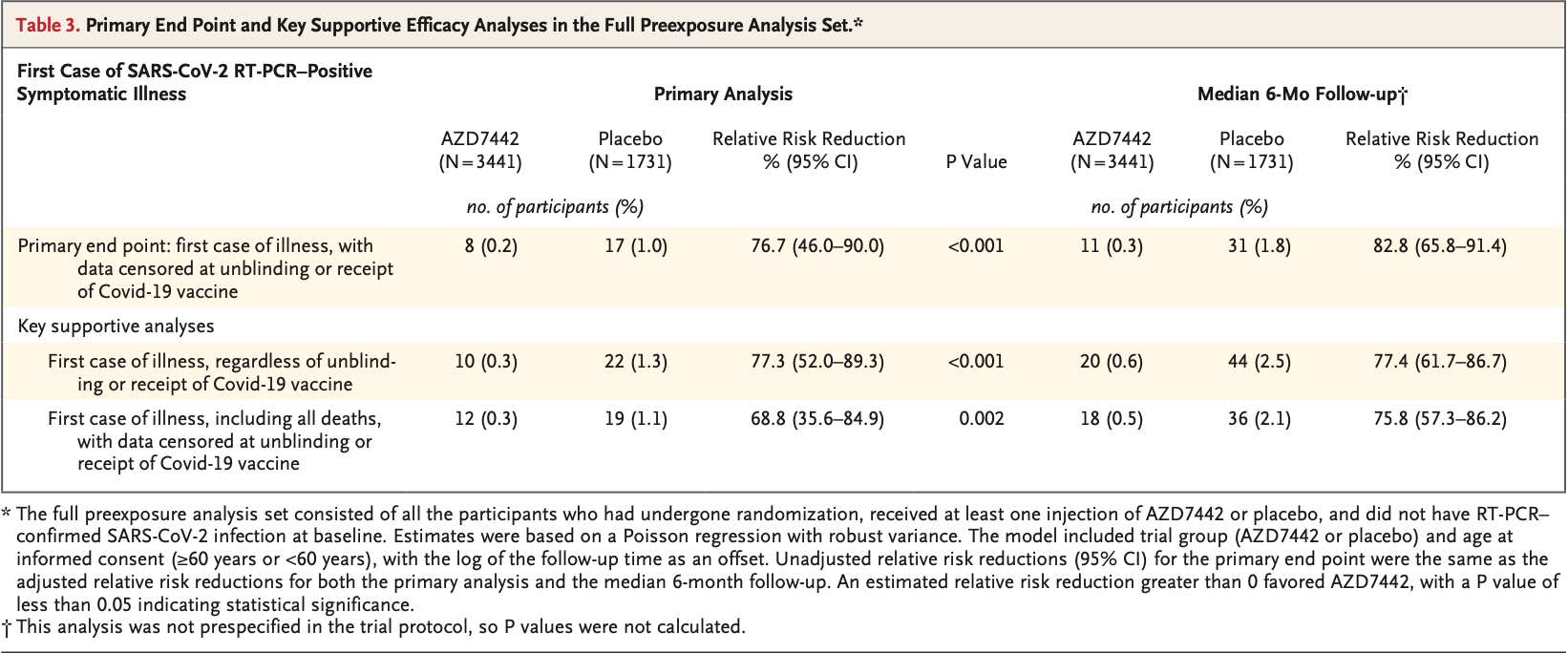
risk of symptomatic case, 82.1% lower, RR 0.18, p < 0.001, treatment 11 of 3,441 (0.3%), control 31 of 1,731 (1.8%), NNT 68, 6 months.
|
|
risk of symptomatic case, 76.3% lower, RR 0.24, p < 0.001, treatment 8 of 3,441 (0.2%), control 17 of 1,731 (1.0%), NNT 133, median 83 days followup.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Planas et al., Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies, bioRxiv, doi:10.1101/2022.11.17.516888.
2.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Levin et al., 20 Apr 2022, Double Blind Randomized Controlled Trial, placebo-controlled, multiple countries, peer-reviewed, 24 authors, study period 21 November, 2020 - 22 March, 2021, trial NCT04625725 (history) (PROVENT).
Contact: mark.esser@astrazeneca.com.
Intramuscular AZD7442 (Tixagevimab–Cilgavimab) for Prevention of Covid-19
New England Journal of Medicine, doi:10.1056/nejmoa2116620
BACKGROUND The monoclonal-antibody combination AZD7442 is composed of tixagevimab and cilgavimab, two neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that have an extended half-life and have been shown to have prophylactic and therapeutic effects in animal models. Pharmacokinetic data in humans indicate that AZD7442 has an extended half-life of approximately 90 days.
METHODS In an ongoing phase 3 trial, we enrolled adults (≥18 years of age) who had an increased risk of an inadequate response to vaccination against coronavirus disease 2019 (Covid-19), an increased risk of exposure to SARS-CoV-2, or both. Participants were randomly assigned in a 2:1 ratio to receive a single dose (two consecutive intramuscular injections, one containing tixagevimab and the other containing cilgavimab) of either 300 mg of AZD7442 or saline placebo, and they were followed for up to 183 days in the primary analysis. The primary safety end point was the incidence of adverse events after a single dose of AZD7442. The primary efficacy end point was symptomatic Covid-19 (SARS-CoV-2 infection confirmed by means of reversetranscriptase-polymerase-chain-reaction assay) occurring after administration of AZD7442 or placebo and on or before day 183.
RESULTS A total of 5197 participants underwent randomization and received one dose of AZD7442 or placebo (3460 in the AZD7442 group and 1737 in the placebo group). The primary analysis was conducted after 30% of the participants had become aware of their randomized assignment. In total, 1221 of 3461 participants (35.3%) in the AZD7442 group and 593 of 1736 participants (34.2%) in the placebo group reported having at least one adverse event, most of which were mild or moderate in severity. Symptomatic Covid-19 occurred in 8 of 3441 participants (0.2%) in the AZD7442 group and in 17 of 1731 participants (1.0%) in the placebo group (relative risk reduction, 76.7%; 95% confidence interval [CI], 46.0 to 90.0; P<0.001); extended follow-up at a median of 6 months showed a relative risk reduction of 82.8% (95% CI, 65.8 to 91.4). Five cases of severe or critical Covid-19 and two Covid-19related deaths occurred, all in the placebo group.
CONCLUSIONS A single dose of AZD7442 had efficacy for the prevention of Covid-19, without evident safety concerns. (Funded by AstraZeneca and the U.S. government; PROVENT ClinicalTrials.gov number, NCT04625725.
SARS-CoV-2 Variants Viral genotypic data collected at illness visits were available for 7 of 11 symptomatic participants in the AZD7442 group and 13 of 31 symptomatic participants in the placebo group. Eleven of these participants were infected with a SARS-CoV-2 vari- A data sharing statement provided by the authors is available with the full text of this article at NEJM.org. We thank the trial participants, their families, and all investigators involved in this trial; the members of the adjudication committee, who assessed deaths that occurred during the trial:
References
Agha, Blake, Chilleo, Wells, Haidar, Suboptimal response to coronavirus disease 2019 messenger RNA vaccines in patients with hematologic malignancies: a need for vigilance in the postmasking era, Open Forum Infect Dis
Bergwerk, Gonen, Lustig, Covid-19 breakthrough infections in vaccinated health care workers, N Engl J Med
Bernal, Andrews, Gower, Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study, BMJ
Cohen, Nirula, Mulligan, Effect of bamlanivimab vs placebo on incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities: a randomized clinical trial, JAMA
Dejnirattisai, Huo, Zhou, SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses, Cell
Dong, Zost, Greaney, Genetic and structural basis for SARS-CoV-2 variant neutralization by a two-antibody cocktail, Nat Microbiol
Dyal, Grant, Broadwater, Covid-19 among workers in meat and poultry processing facilities -19 states, April 2020, MMWR Morb Mortal Wkly Rep
Haas, Angulo, Mclaughlin, Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data, Lancet
Haberman, Herati, Simon, Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease, Ann Rheum Dis
Iketani, Liu, Guo, Antibody evasion properties of SARS-CoV-2 Omicron sublineages, Nature
Kamar, Abravanel, Marion, Couat, Izopet et al., Three doses of an mRNA Covid-19 vaccine in solidorgan transplant recipients, N Engl J Med
Kearns, Siebert, Willicombe, Examining the immunological effects of COVID-19 vaccination in patients with conditions potentially leading to diminished immune response capacity -the OCTAVE trial, doi:10.2139/ssrn.3910058
Kennedy, Lin, Goodhand, Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD, Gut
Kirby, Evidence mounts on the disproportionate effect of COVID-19 on ethnic minorities, Lancet Respir Med
Medeiros-Ribeiro, Aikawa, Saad, Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial, Nat Med
Narasimhan, Mahimainathan, Clark, Serological response in lung transplant recipients after two doses of SARS-CoV-2 mRNA vaccines, Vaccines
Nasreen, Chung, He, Effectiveness of COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe outcomes with variants of concern in Ontario, Nat Microbiol
O'brien, Forleo-Neto, Musser, Subcutaneous REGEN-COV antibody combination to prevent Covid-19
Oganesyan, Gao, Shirinian, Wu, Dall et al., The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in nonhuman primates and has an extended half-life in humans, Acta Crystallogr D Biol Crystallogr
Pelfrene, Mura, Sanches, Cavaleri, Monoclonal antibodies as anti-infective products: a promising future?
Pritchard, Matthews, Stoesser, Impact of vaccination on new SARS-CoV-2 infections in the United Kingdom, Nat Med
Robbie, Criste, Dall, Wf, A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults, Antimicrob Agents Chemother
Tai, Shah, Doubeni, Sia, Wieland, The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States, Clin Infect Dis
Taylor, Adams, Hufford, De La Torre, Winthrop et al., Neutralizing monoclonal antibodies for treatment of COVID-19, Nat Rev Immunol
Taylor, Wall, Ross, Cross sectional investigation of a COVID-19 outbreak at a London army barracks: neutralising antibodies and virus isolation, Lancet Reg Health Eur
Vanblargan, Errico, Halfmann, An infectious SARS-CoV-2
Zhou, Wang, Misasi, Structural basis for potent antibody neutralization of SARS-CoV-2 variants including B, Science
Zost, Gilchuk, Case, Potently neutralizing and protective human antibodies against SARS-CoV-2, Nature
Zost, Gilchuk, Chen, Rapid isolation and profiling of a diverse panel of human monoclonal antibodies targeting the SARS-CoV-2 spike protein, Nat Med
Zou, A modified Poisson regression approach to prospective studies with binary data, Am J Epidemiol
DOI record:
{
"DOI": "10.1056/nejmoa2116620",
"ISSN": [
"0028-4793",
"1533-4406"
],
"URL": "http://dx.doi.org/10.1056/nejmoa2116620",
"alternative-id": [
"10.1056/NEJMoa2116620"
],
"author": [
{
"affiliation": [
{
"name": "From the University of Colorado School of Medicine, Aurora (M.J.L.); North Manchester General Hospital, Manchester (A.U.), Biometrics (A.T., S.S.) and Clinical Development (R.B., G.C.K.W.K.), Vaccines and Immune Therapies, Biopharmaceuticals Research and Development (M.N.P.), AstraZeneca, Cambridge, and Mounts Bay Medical, Penzance (A.E.) — all in the United Kingdom; the Division of Infectious Diseases, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels (S.D.W.); Université de..."
}
],
"family": "Levin",
"given": "Myron J.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "From the University of Colorado School of Medicine, Aurora (M.J.L.); North Manchester General Hospital, Manchester (A.U.), Biometrics (A.T., S.S.) and Clinical Development (R.B., G.C.K.W.K.), Vaccines and Immune Therapies, Biopharmaceuticals Research and Development (M.N.P.), AstraZeneca, Cambridge, and Mounts Bay Medical, Penzance (A.E.) — all in the United Kingdom; the Division of Infectious Diseases, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels (S.D.W.); Université de..."
}
],
"family": "Ustianowski",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Colorado School of Medicine, Aurora (M.J.L.); North Manchester General Hospital, Manchester (A.U.), Biometrics (A.T., S.S.) and Clinical Development (R.B., G.C.K.W.K.), Vaccines and Immune Therapies, Biopharmaceuticals Research and Development (M.N.P.), AstraZeneca, Cambridge, and Mounts Bay Medical, Penzance (A.E.) — all in the United Kingdom; the Division of Infectious Diseases, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels (S.D.W.); Université de..."
}
],
"family": "De Wit",
"given": "Stéphane",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Colorado School of Medicine, Aurora (M.J.L.); North Manchester General Hospital, Manchester (A.U.), Biometrics (A.T., S.S.) and Clinical Development (R.B., G.C.K.W.K.), Vaccines and Immune Therapies, Biopharmaceuticals Research and Development (M.N.P.), AstraZeneca, Cambridge, and Mounts Bay Medical, Penzance (A.E.) — all in the United Kingdom; the Division of Infectious Diseases, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels (S.D.W.); Université de..."
}
],
"family": "Launay",
"given": "Odile",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Colorado School of Medicine, Aurora (M.J.L.); North Manchester General Hospital, Manchester (A.U.), Biometrics (A.T., S.S.) and Clinical Development (R.B., G.C.K.W.K.), Vaccines and Immune Therapies, Biopharmaceuticals Research and Development (M.N.P.), AstraZeneca, Cambridge, and Mounts Bay Medical, Penzance (A.E.) — all in the United Kingdom; the Division of Infectious Diseases, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels (S.D.W.); Université de..."
}
],
"family": "Avila",
"given": "Miles",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Colorado School of Medicine, Aurora (M.J.L.); North Manchester General Hospital, Manchester (A.U.), Biometrics (A.T., S.S.) and Clinical Development (R.B., G.C.K.W.K.), Vaccines and Immune Therapies, Biopharmaceuticals Research and Development (M.N.P.), AstraZeneca, Cambridge, and Mounts Bay Medical, Penzance (A.E.) — all in the United Kingdom; the Division of Infectious Diseases, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels (S.D.W.); Université de..."
}
],
"family": "Templeton",
"given": "Alison",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Colorado School of Medicine, Aurora (M.J.L.); North Manchester General Hospital, Manchester (A.U.), Biometrics (A.T., S.S.) and Clinical Development (R.B., G.C.K.W.K.), Vaccines and Immune Therapies, Biopharmaceuticals Research and Development (M.N.P.), AstraZeneca, Cambridge, and Mounts Bay Medical, Penzance (A.E.) — all in the United Kingdom; the Division of Infectious Diseases, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels (S.D.W.); Université de..."
}
],
"family": "Yuan",
"given": "Yuan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Colorado School of Medicine, Aurora (M.J.L.); North Manchester General Hospital, Manchester (A.U.), Biometrics (A.T., S.S.) and Clinical Development (R.B., G.C.K.W.K.), Vaccines and Immune Therapies, Biopharmaceuticals Research and Development (M.N.P.), AstraZeneca, Cambridge, and Mounts Bay Medical, Penzance (A.E.) — all in the United Kingdom; the Division of Infectious Diseases, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels (S.D.W.); Université de..."
}
],
"family": "Seegobin",
"given": "Seth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Colorado School of Medicine, Aurora (M.J.L.); North Manchester General Hospital, Manchester (A.U.), Biometrics (A.T., S.S.) and Clinical Development (R.B., G.C.K.W.K.), Vaccines and Immune Therapies, Biopharmaceuticals Research and Development (M.N.P.), AstraZeneca, Cambridge, and Mounts Bay Medical, Penzance (A.E.) — all in the United Kingdom; the Division of Infectious Diseases, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels (S.D.W.); Université de..."
}
],
"family": "Ellery",
"given": "Adam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Colorado School of Medicine, Aurora (M.J.L.); North Manchester General Hospital, Manchester (A.U.), Biometrics (A.T., S.S.) and Clinical Development (R.B., G.C.K.W.K.), Vaccines and Immune Therapies, Biopharmaceuticals Research and Development (M.N.P.), AstraZeneca, Cambridge, and Mounts Bay Medical, Penzance (A.E.) — all in the United Kingdom; the Division of Infectious Diseases, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels (S.D.W.); Université de..."
}
],
"family": "Levinson",
"given": "Dennis J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Colorado School of Medicine, Aurora (M.J.L.); North Manchester General Hospital, Manchester (A.U.), Biometrics (A.T., S.S.) and Clinical Development (R.B., G.C.K.W.K.), Vaccines and Immune Therapies, Biopharmaceuticals Research and Development (M.N.P.), AstraZeneca, Cambridge, and Mounts Bay Medical, Penzance (A.E.) — all in the United Kingdom; the Division of Infectious Diseases, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels (S.D.W.); Université de..."
}
],
"family": "Ambery",
"given": "Philip",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Colorado School of Medicine, Aurora (M.J.L.); North Manchester General Hospital, Manchester (A.U.), Biometrics (A.T., S.S.) and Clinical Development (R.B., G.C.K.W.K.), Vaccines and Immune Therapies, Biopharmaceuticals Research and Development (M.N.P.), AstraZeneca, Cambridge, and Mounts Bay Medical, Penzance (A.E.) — all in the United Kingdom; the Division of Infectious Diseases, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels (S.D.W.); Université de..."
}
],
"family": "Arends",
"given": "Rosalinda H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Colorado School of Medicine, Aurora (M.J.L.); North Manchester General Hospital, Manchester (A.U.), Biometrics (A.T., S.S.) and Clinical Development (R.B., G.C.K.W.K.), Vaccines and Immune Therapies, Biopharmaceuticals Research and Development (M.N.P.), AstraZeneca, Cambridge, and Mounts Bay Medical, Penzance (A.E.) — all in the United Kingdom; the Division of Infectious Diseases, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels (S.D.W.); Université de..."
}
],
"family": "Beavon",
"given": "Rohini",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Colorado School of Medicine, Aurora (M.J.L.); North Manchester General Hospital, Manchester (A.U.), Biometrics (A.T., S.S.) and Clinical Development (R.B., G.C.K.W.K.), Vaccines and Immune Therapies, Biopharmaceuticals Research and Development (M.N.P.), AstraZeneca, Cambridge, and Mounts Bay Medical, Penzance (A.E.) — all in the United Kingdom; the Division of Infectious Diseases, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels (S.D.W.); Université de..."
}
],
"family": "Dey",
"given": "Kanika",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Colorado School of Medicine, Aurora (M.J.L.); North Manchester General Hospital, Manchester (A.U.), Biometrics (A.T., S.S.) and Clinical Development (R.B., G.C.K.W.K.), Vaccines and Immune Therapies, Biopharmaceuticals Research and Development (M.N.P.), AstraZeneca, Cambridge, and Mounts Bay Medical, Penzance (A.E.) — all in the United Kingdom; the Division of Infectious Diseases, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels (S.D.W.); Université de..."
}
],
"family": "Garbes",
"given": "Pedro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Colorado School of Medicine, Aurora (M.J.L.); North Manchester General Hospital, Manchester (A.U.), Biometrics (A.T., S.S.) and Clinical Development (R.B., G.C.K.W.K.), Vaccines and Immune Therapies, Biopharmaceuticals Research and Development (M.N.P.), AstraZeneca, Cambridge, and Mounts Bay Medical, Penzance (A.E.) — all in the United Kingdom; the Division of Infectious Diseases, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels (S.D.W.); Université de..."
}
],
"family": "Kelly",
"given": "Elizabeth J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Colorado School of Medicine, Aurora (M.J.L.); North Manchester General Hospital, Manchester (A.U.), Biometrics (A.T., S.S.) and Clinical Development (R.B., G.C.K.W.K.), Vaccines and Immune Therapies, Biopharmaceuticals Research and Development (M.N.P.), AstraZeneca, Cambridge, and Mounts Bay Medical, Penzance (A.E.) — all in the United Kingdom; the Division of Infectious Diseases, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels (S.D.W.); Université de..."
}
],
"family": "Koh",
"given": "Gavin C.K.W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Colorado School of Medicine, Aurora (M.J.L.); North Manchester General Hospital, Manchester (A.U.), Biometrics (A.T., S.S.) and Clinical Development (R.B., G.C.K.W.K.), Vaccines and Immune Therapies, Biopharmaceuticals Research and Development (M.N.P.), AstraZeneca, Cambridge, and Mounts Bay Medical, Penzance (A.E.) — all in the United Kingdom; the Division of Infectious Diseases, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels (S.D.W.); Université de..."
}
],
"family": "Near",
"given": "Karen A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Colorado School of Medicine, Aurora (M.J.L.); North Manchester General Hospital, Manchester (A.U.), Biometrics (A.T., S.S.) and Clinical Development (R.B., G.C.K.W.K.), Vaccines and Immune Therapies, Biopharmaceuticals Research and Development (M.N.P.), AstraZeneca, Cambridge, and Mounts Bay Medical, Penzance (A.E.) — all in the United Kingdom; the Division of Infectious Diseases, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels (S.D.W.); Université de..."
}
],
"family": "Padilla",
"given": "Kelly W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Colorado School of Medicine, Aurora (M.J.L.); North Manchester General Hospital, Manchester (A.U.), Biometrics (A.T., S.S.) and Clinical Development (R.B., G.C.K.W.K.), Vaccines and Immune Therapies, Biopharmaceuticals Research and Development (M.N.P.), AstraZeneca, Cambridge, and Mounts Bay Medical, Penzance (A.E.) — all in the United Kingdom; the Division of Infectious Diseases, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels (S.D.W.); Université de..."
}
],
"family": "Psachoulia",
"given": "Konstantina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Colorado School of Medicine, Aurora (M.J.L.); North Manchester General Hospital, Manchester (A.U.), Biometrics (A.T., S.S.) and Clinical Development (R.B., G.C.K.W.K.), Vaccines and Immune Therapies, Biopharmaceuticals Research and Development (M.N.P.), AstraZeneca, Cambridge, and Mounts Bay Medical, Penzance (A.E.) — all in the United Kingdom; the Division of Infectious Diseases, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels (S.D.W.); Université de..."
}
],
"family": "Sharbaugh",
"given": "Audrey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Colorado School of Medicine, Aurora (M.J.L.); North Manchester General Hospital, Manchester (A.U.), Biometrics (A.T., S.S.) and Clinical Development (R.B., G.C.K.W.K.), Vaccines and Immune Therapies, Biopharmaceuticals Research and Development (M.N.P.), AstraZeneca, Cambridge, and Mounts Bay Medical, Penzance (A.E.) — all in the United Kingdom; the Division of Infectious Diseases, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels (S.D.W.); Université de..."
}
],
"family": "Streicher",
"given": "Katie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Colorado School of Medicine, Aurora (M.J.L.); North Manchester General Hospital, Manchester (A.U.), Biometrics (A.T., S.S.) and Clinical Development (R.B., G.C.K.W.K.), Vaccines and Immune Therapies, Biopharmaceuticals Research and Development (M.N.P.), AstraZeneca, Cambridge, and Mounts Bay Medical, Penzance (A.E.) — all in the United Kingdom; the Division of Infectious Diseases, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels (S.D.W.); Université de..."
}
],
"family": "Pangalos",
"given": "Menelas N.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the University of Colorado School of Medicine, Aurora (M.J.L.); North Manchester General Hospital, Manchester (A.U.), Biometrics (A.T., S.S.) and Clinical Development (R.B., G.C.K.W.K.), Vaccines and Immune Therapies, Biopharmaceuticals Research and Development (M.N.P.), AstraZeneca, Cambridge, and Mounts Bay Medical, Penzance (A.E.) — all in the United Kingdom; the Division of Infectious Diseases, Saint-Pierre University Hospital, Université Libre de Bruxelles, Brussels (S.D.W.); Université de..."
}
],
"family": "Esser",
"given": "Mark T.",
"sequence": "additional"
}
],
"container-title": [
"New England Journal of Medicine"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
4,
20
]
],
"date-time": "2022-04-20T21:02:26Z",
"timestamp": 1650488546000
},
"deposited": {
"date-parts": [
[
2022,
4,
20
]
],
"date-time": "2022-04-20T21:02:29Z",
"timestamp": 1650488549000
},
"funder": [
{
"DOI": "10.13039/100000016",
"award": [
"W911QY-21-9-0001"
],
"doi-asserted-by": "publisher",
"name": "U.S. Department of Health and Human Services"
},
{
"DOI": "10.13039/100000005",
"award": [
"W911QY-21-9-0001"
],
"doi-asserted-by": "publisher",
"name": "U.S. Department of Defense"
},
{
"DOI": "10.13039/100004325",
"doi-asserted-by": "publisher",
"name": "AstraZeneca"
}
],
"indexed": {
"date-parts": [
[
2022,
4,
21
]
],
"date-time": "2022-04-21T21:15:46Z",
"timestamp": 1650575746161
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "0028-4793"
},
{
"type": "electronic",
"value": "1533-4406"
}
],
"issued": {
"date-parts": [
[
2022,
4,
20
]
]
},
"language": "en",
"license": [
{
"URL": "http://www.nejmgroup.org/legal/terms-of-use.htm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
4,
20
]
],
"date-time": "2022-04-20T00:00:00Z",
"timestamp": 1650412800000
}
}
],
"link": [
{
"URL": "http://www.nejm.org/doi/pdf/10.1056/NEJMoa2116620",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "150",
"original-title": [],
"prefix": "10.1056",
"published": {
"date-parts": [
[
2022,
4,
20
]
]
},
"published-online": {
"date-parts": [
[
2022,
4,
20
]
]
},
"publisher": "Massachusetts Medical Society",
"reference-count": 25,
"references-count": 25,
"relation": {},
"resource": {
"primary": {
"URL": "http://www.nejm.org/doi/10.1056/NEJMoa2116620"
}
},
"score": 1,
"short-container-title": [
"N Engl J Med"
],
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": [
"Intramuscular AZD7442 (Tixagevimab–Cilgavimab) for Prevention of Covid-19"
],
"type": "journal-article"
}