Favipiravir In Adults with Moderate to Severe COVID-19: A Phase 3 Multicentre, Randomized, Double-Blind, Placebo-Controlled Trial
et al., medRxiv, doi:10.1101/2021.11.08.21265884, NCT04529499, Nov 2021
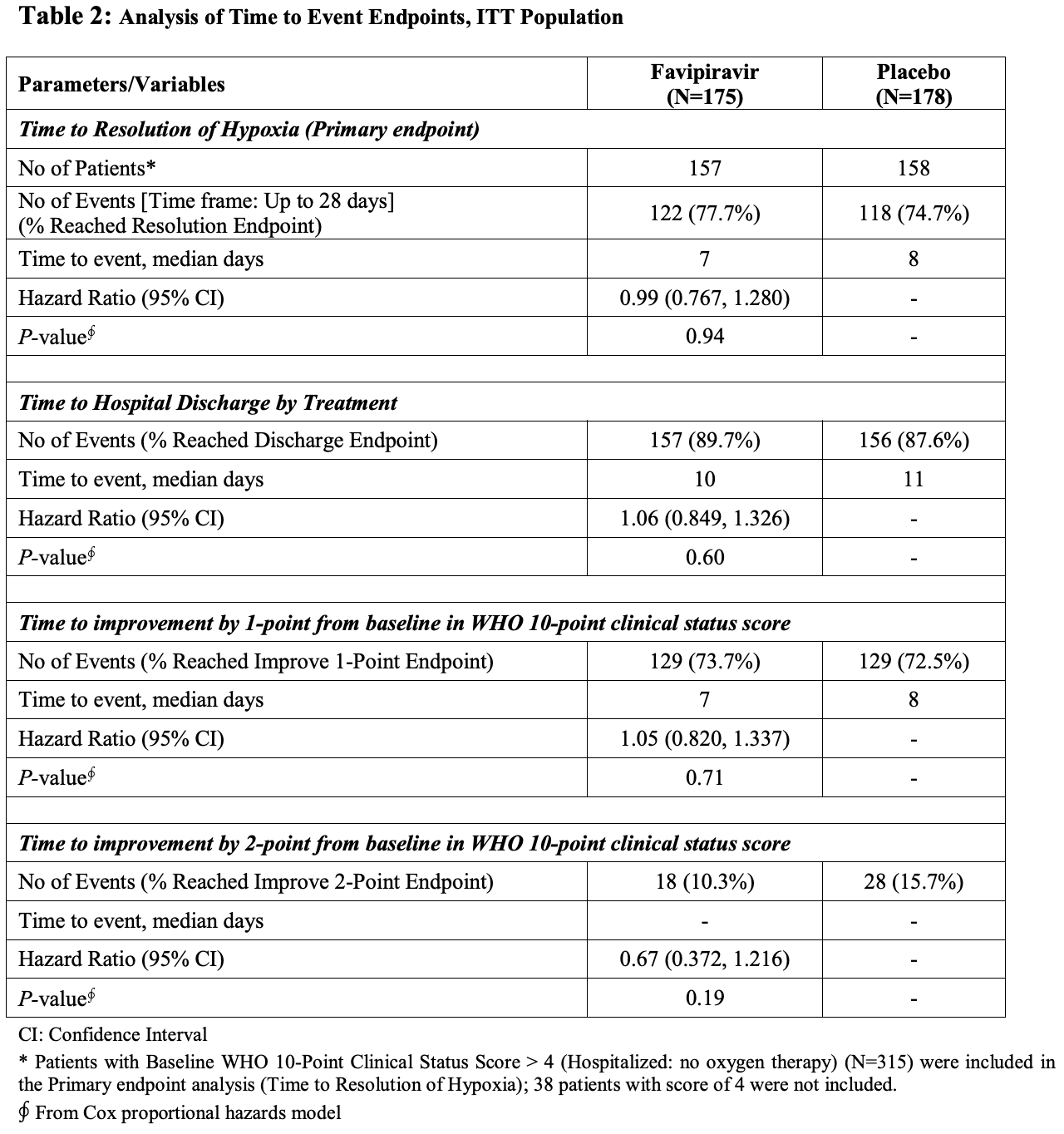
Late stage RCT with 353 hospitalized patients, showing no significant differences with favipiravir treatment overall, however a trend towards benefit was seen within patients treated relatively early, including a statistically significant shorter time to discharge with treatment.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
|
risk of death, 29.5% higher, RR 1.29, p = 0.54, treatment 14 of 175 (8.0%), control 11 of 178 (6.2%), day 28.
|
|
risk of mechanical ventilation, 33.0% higher, RR 1.33, p = 0.54, treatment 17 of 175 (9.7%), control 13 of 178 (7.3%).
|
|
risk of ICU admission, 1.7% higher, RR 1.02, p = 0.54, treatment 20 of 175 (11.4%), control 20 of 178 (11.2%).
|
|
time to resolution of hypoxia, 1.0% higher, HR 1.01, p = 0.94, treatment 157, control 158, inverted to make HR<1 favor treatment, primary outcome.
|
|
time to hospital discharge, 5.7% lower, HR 0.94, p = 0.60, treatment 175, control 178, inverted to make HR<1 favor treatment.
|
|
time to resolution of hypoxia, 17.4% lower, HR 0.83, p = 0.29, treatment 157, control 158, inverted to make HR<1 favor treatment, earlier treatment subgroup, primary outcome.
|
|
time to hospital discharge, 32.0% lower, HR 0.68, p = 0.01, treatment 175, control 178, inverted to make HR<1 favor treatment, earlier treatment subgroup.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Shenoy et al., 9 Nov 2021, Double Blind Randomized Controlled Trial, Kuwait, preprint, 8 authors, study period 22 August, 2020 - 27 January, 2021, average treatment delay 6.3 days, trial NCT04529499 (history).
Favipiravir In Adults with Moderate to Severe COVID-19: A Phase 3 Multicentre, Randomized, Double-Blinded, Placebo-Controlled Trial
doi:10.1101/2021.11.08.21265884
Aim: To assess the efficacy and safety of favipiravir in adults with moderate to severe coronavirus disease 2019 . Methods: In this randomized, double-blind, multicenter, phase 3 trial, adults (21-80 years) with real-time reverse transcriptase polymerase chain reaction (rRT-PCR) confirmed SARS-CoV-2 infection and presenting with moderate to severe COVID-19 and requiring hospitalization were randomized 1:1 to oral favipiravir (day 1: 1800 mg BID and days 2-10: 800 mg BID) (FPV) plus standard supportive care (SoC) versus placebo plus SoC (placebo). The primary endpoint was time to resolution of hypoxia. Results: In total, 353 patients were randomized to receive either FPV or placebo (175 and 178 in the FPV and placebo groups, respectively). Overall, 76% of the patients (240/315, 78% in FPV vs. 75% in placebo group) reached resolution of hypoxia on or before day 28. The median time to resolution of hypoxia was 7 days in the FPV group and 8 days in the placebo group. Treatment effect was not significant [Hazard ratio (HR) (95% CI): 0.991 (0.767, 1.280) (p=0.94)]. Patients in the lower NEWS-2 clinical risk subgroup were more likely to achieve shorter time to resolution of hypoxia with the median time to resolution of hypoxia of 6 days in FPV and 7 days in placebo group [HR (95% CI): 1.21 (0.847, 1.731) (p=0.29)]; shorter time to hospital discharge with a median time to discharge of 8 and 10 days in the FPV and placebo group, respectively [HR (95% CI): 1.47 (1.081, 1.997) (p=0.014)]; and shorter time to improvement by 1-point improvement over baseline in WHO 10-point clinical status score with the median time to improvement by 1-point from baseline of 6 and 7 days in the FPV and placebo group, respectively [HR (95% CI): 1.16 (0.830, 1.624) (p=0.38)] than higher NEWS-2 clinical risk subgroup. Treatment emergent adverse event (TEAEs) were experienced by 62/334 (19%) patients [35/168 (21%) patients in FPV and 27/166 (16%) in placebo group]. Hyperuricaemia/increased blood uric acid was reported in 9 (3%)/2 (1%) patients [8 (5%)/1(1%) patients in FPV and 1 (1%)/1(1%) in placebo group] ,which were of mild intensity and transient. Overall, 36 serious adverse events (SAEs) were reported, 20 in FPV and 16 in placebo group.
Client Confidential
Conclusion: The trial did not find favipiravir to be effective in moderate to severe, hospitalized COVID-19 patients; favourable clinical trends were observed in patients with lower NEWS-2 risk when early administration of favipiravir could be achieved.
Conflict of Interest Drs. Srinivas Shenoy and Sagar Munjal are paid employees of Dr. Reddy's Laboratories and didn't receive any additional compensation for this study. Dr Salman Al-Sabah was the Principal Investigator and National Co-Ordinator (Kuwait) for the trial, while Drs. Sarah Al Youha, Mohammad Alghounaim, Sulaiman Almazeedi and Yousef Alshamali were sub-Investigators. The Investigators and sub-investigators are employees of various public sector hospitals in the State of Kuwait and did not receive any financial compensation for this study. Dr Richard H Kaczynski is a consultant to Fujifilm Toyoma Chemical Co. Ltd. and Chief Medical Officer of AiPharma. He contributed significantly to study design and did not receive any additional compensation for this study.
References
Bavaniya, Favipiravir for Patients With Mild to Moderate Disease From Novel Coronavirus
Boretti, Favipiravir use for SARS CoV-2 infection, Pharmacol Rep PR, doi:10.1007/s43440-020-00175-2
Burki, Challenges in the rollout of COVID-19 vaccines worldwide, Lancet Respir Med, doi:10.1016/S2213-2600(21)00129-6
Callaway, Delta coronavirus variant: scientists brace for impact, Nature, doi:10.1038/d41586-021-01696-3
Chen, Zhang, Huang, Favipiravir Versus Arbidol for Clinical Recovery Rate in Moderate and Severe Adult COVID-19 Patients: A Prospective, Multicenter, Open-Label, Randomized Controlled Clinical Trial, Front Pharmacol, doi:10.3389/fphar.2021.683296
Chung, Thone, Kwon, COVID-19 vaccines: The status and perspectives in delivery points of view, Adv Drug Deliv Rev, doi:10.1016/j.addr.2020.12.011
Dabbous, Abd-Elsalam, El-Sayed, Efficacy of favipiravir in COVID-19 treatment: a multi-center randomized study, Arch Virol, doi:10.1007/s00705-021-04956-9
Doi, Hibino, Hase, A Prospective, Randomized, Open-Label Trial of Early versus Late Favipiravir Therapy in Hospitalized Patients with COVID-19, Antimicrob Agents Chemother, doi:10.1128/AAC.01897-20
Doi, Kondo, Matsuyama, Preliminary Report of the Favipiravir Observational Study in Japan
Furuta, Gowen, Takahashi, Favipiravir (T-705), a novel viral RNA polymerase inhibitor, Antiviral Res, doi:10.1016/j.antiviral.2013.09.015
Furuta, Komeno, Nakamura, Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase, Proc Jpn Acad Ser B Phys Biol Sci, doi:10.2183/pjab.93.027
Hartmann, Terao, Garattini, The Impact of Single Nucleotide Polymorphisms on Human Aldehyde Oxidase, Drug Metab Dispos, doi:10.1124/dmd.111.043828
Mccullough, Favipiravir and the Need for Early Ambulatory Treatment of SARS-CoV-2 Infection (COVID-19), Antimicrob Agents Chemother, doi:10.1128/AAC.02017-20
Mishima, Anzai, Miyazaki, Uric Acid Elevation by Favipiravir, an Antiviral Drug, Tohoku J Exp Med, doi:10.1620/tjem.251.87
Nicola, Neill, Sohrabi, Evidence based management guideline for the COVID-19 pandemic -Review article, Int J Surg Lond Engl, doi:10.1016/j.ijsu.2020.04.001
Oestereich, Lüdtke, Wurr, Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model, Antiviral Res, doi:10.1016/j.antiviral.2014.02.014
Pan, Peto, Repurposed Antiviral Drugs for Covid-19 -Interim WHO Solidarity Trial Results, N Engl J Med, doi:10.1056/NEJMoa2023184
Shrestha, Budhathoki, Khadka, Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis, Virol J, doi:10.1186/s12985-020-01412-z
Smither, Eastaugh, Steward, Post-exposure efficacy of oral T-705 (Favipiravir) against inhalational Ebola virus infection in a mouse model, Antiviral Res, doi:10.1016/j.antiviral.2014.01.012
Udwadia, Singh, Barkate, Efficacy and safety of favipiravir, an oral RNAdependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial, Int J Infect Dis IJID Off Publ Int Soc Infect Dis, doi:10.1016/j.ijid.2020.11.142
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res, doi:10.1038/s41422-020-0282-0
Yu, Tian, Chu, COVID-19 patients benefit from early antiviral treatment: A comparative, retrospective study, J Med Virol, doi:10.1002/jmv.26129
Łagocka, Dziedziejko, Kłos, Favipiravir in Therapy of Viral Infections, J Clin Med, doi:10.3390/jcm10020273
DOI record:
{
"DOI": "10.1101/2021.11.08.21265884",
"URL": "http://dx.doi.org/10.1101/2021.11.08.21265884",
"abstract": "<jats:title>ABSTRACT</jats:title><jats:sec><jats:title>Aim</jats:title><jats:p>To assess the efficacy and safety of favipiravir in adults with moderate to severe coronavirus disease 2019 (COVID-19).</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>In this randomized, double-blind, multicenter, phase 3 trial, adults (21-80 years) with real-time reverse transcriptase polymerase chain reaction (rRT-PCR) confirmed SARS-CoV-2 infection and presenting with moderate to severe COVID-19 and requiring hospitalization were randomized 1:1 to oral favipiravir (day 1: 1800 mg BID and days 2-10: 800 mg BID) (FPV) plus standard supportive care (SoC) versus placebo plus SoC (placebo). The primary endpoint was time to resolution of hypoxia.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>In total, 353 patients were randomized to receive either FPV or placebo (175 and 178 in the FPV and placebo groups, respectively). Overall, 76% of the patients (240/315, 78% in FPV vs. 75% in placebo group) reached resolution of hypoxia on or before day 28. The median time to resolution of hypoxia was 7 days in the FPV group and 8 days in the placebo group. Treatment effect was not significant [Hazard ratio (HR) (95% CI): 0.991 (0.767, 1.280) (<jats:italic>p</jats:italic>=0.94)].</jats:p><jats:p>Patients in the lower NEWS-2 clinical risk subgroup were more likely to achieve shorter time to resolution of hypoxia with the median time to resolution of hypoxia of 6 days in FPV and 7 days in placebo group [HR (95% CI): 1.21 (0.847, 1.731) (<jats:italic>p</jats:italic>=0.29)]; shorter time to hospital discharge with a median time to discharge of 8 and 10 days in the FPV and placebo group, respectively [HR (95% CI): 1.47 (1.081, 1.997) (<jats:italic>p=</jats:italic>0.014)]; and shorter time to improvement by 1-point improvement over baseline in WHO 10-point clinical status score with the median time to improvement by 1-point from baseline of 6 and 7 days in the FPV and placebo group, respectively [HR (95% CI): 1.16 (0.830, 1.624) (<jats:italic>p=</jats:italic>0.38)] than higher NEWS-2 clinical risk subgroup.</jats:p><jats:p>Treatment emergent adverse event (TEAEs) were experienced by 62/334 (19%) patients [35/168 (21%) patients in FPV and 27/166 (16%) in placebo group]. Hyperuricaemia/increased blood uric acid was reported in 9 (3%)/2 (1%) patients [8 (5%)/1(1%) patients in FPV and 1 (1%)/1(1%) in placebo group], which were of mild intensity and transient. Overall, 36 serious adverse events (SAEs) were reported, 20 in FPV and 16 in placebo group.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>The trial did not find favipiravir to be effective in moderate to severe, hospitalized COVID-19 patients; favourable clinical trends were observed in patients with lower NEWS-2 risk when early administration of favipiravir could be achieved.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2021,
11,
9
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0002-0147-7148",
"affiliation": [],
"authenticated-orcid": false,
"family": "Shenoy",
"given": "Srinivas",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-5387-720X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Munjal",
"given": "Sagar",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1900-0150",
"affiliation": [],
"authenticated-orcid": false,
"family": "Youha",
"given": "Sarah Al",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0665-3761",
"affiliation": [],
"authenticated-orcid": false,
"family": "Alghounaim",
"given": "Mohammad",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0686-0664",
"affiliation": [],
"authenticated-orcid": false,
"family": "Almazeedi",
"given": "Sulaiman",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1948-1559",
"affiliation": [],
"authenticated-orcid": false,
"family": "Alshamali",
"given": "Yousef",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1459-7085",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kaszynski",
"given": "Richard H",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8081-8544",
"affiliation": [],
"authenticated-orcid": false,
"family": "Al-Sabah",
"given": "Salman",
"sequence": "additional"
},
{
"affiliation": [],
"name": "Kuwait Clinical Trial Group",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
11,
9
]
],
"date-time": "2021-11-09T19:15:15Z",
"timestamp": 1636485315000
},
"deposited": {
"date-parts": [
[
2021,
11,
12
]
],
"date-time": "2021-11-12T06:45:21Z",
"timestamp": 1636699521000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2024,
3,
28
]
],
"date-time": "2024-03-28T04:44:52Z",
"timestamp": 1711601092600
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 4,
"issued": {
"date-parts": [
[
2021,
11,
9
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.11.08.21265884",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
11,
9
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
11,
9
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1016/j.ijsu.2020.04.001",
"doi-asserted-by": "publisher",
"key": "2021111122450686000_2021.11.08.21265884v1.1"
},
{
"DOI": "10.1016/j.addr.2020.12.011",
"doi-asserted-by": "publisher",
"key": "2021111122450686000_2021.11.08.21265884v1.2"
},
{
"DOI": "10.1016/S2213-2600(21)00129-6",
"doi-asserted-by": "publisher",
"key": "2021111122450686000_2021.11.08.21265884v1.3"
},
{
"DOI": "10.1038/d41586-021-01696-3",
"doi-asserted-by": "publisher",
"key": "2021111122450686000_2021.11.08.21265884v1.4"
},
{
"DOI": "10.1056/NEJMoa2023184",
"doi-asserted-by": "publisher",
"key": "2021111122450686000_2021.11.08.21265884v1.5"
},
{
"DOI": "10.2183/pjab.93.027",
"doi-asserted-by": "publisher",
"key": "2021111122450686000_2021.11.08.21265884v1.6"
},
{
"DOI": "10.1016/j.antiviral.2014.01.012",
"doi-asserted-by": "publisher",
"key": "2021111122450686000_2021.11.08.21265884v1.7"
},
{
"DOI": "10.1016/j.antiviral.2013.09.015",
"doi-asserted-by": "publisher",
"key": "2021111122450686000_2021.11.08.21265884v1.8"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"doi-asserted-by": "publisher",
"key": "2021111122450686000_2021.11.08.21265884v1.9"
},
{
"DOI": "10.1016/j.antiviral.2014.02.014",
"doi-asserted-by": "publisher",
"key": "2021111122450686000_2021.11.08.21265884v1.10"
},
{
"key": "2021111122450686000_2021.11.08.21265884v1.11",
"unstructured": "Favipiravir Observational Study Interim Report 3. 2021;:6."
},
{
"DOI": "10.3390/jcm10020273",
"doi-asserted-by": "publisher",
"key": "2021111122450686000_2021.11.08.21265884v1.12"
},
{
"key": "2021111122450686000_2021.11.08.21265884v1.13",
"unstructured": "National Early Warning Score (NEWS) 2. RCP Lond. 2017. https://www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2 (accessed 2 Nov 2021)."
},
{
"key": "2021111122450686000_2021.11.08.21265884v1.14",
"unstructured": "U.S. Department of Health and Human Services, Food and Drug Administration. COVID-19: Developing Drugs and Biological Products for Treatment or Prevention. 2021;:26."
},
{
"key": "2021111122450686000_2021.11.08.21265884v1.15",
"unstructured": "Prasann Bavaniya. Favipiravir for Patients With Mild to Moderate Disease From Novel Coronavirus (COVID-19). http://clinicaltrials.gov 2021. https://clinicaltrials.gov/ct2/show/NCT04600895 (accessed 31 Oct 2021)."
},
{
"DOI": "10.1128/AAC.02017-20",
"doi-asserted-by": "publisher",
"key": "2021111122450686000_2021.11.08.21265884v1.16"
},
{
"DOI": "10.1128/AAC.01897-20",
"doi-asserted-by": "publisher",
"key": "2021111122450686000_2021.11.08.21265884v1.17"
},
{
"DOI": "10.1002/jmv.26129",
"doi-asserted-by": "publisher",
"key": "2021111122450686000_2021.11.08.21265884v1.18"
},
{
"DOI": "10.1007/s00705-021-04956-9",
"doi-asserted-by": "publisher",
"key": "2021111122450686000_2021.11.08.21265884v1.19"
},
{
"DOI": "10.1007/s43440-020-00175-2",
"doi-asserted-by": "publisher",
"key": "2021111122450686000_2021.11.08.21265884v1.20"
},
{
"DOI": "10.1186/s12985-020-01412-z",
"doi-asserted-by": "publisher",
"key": "2021111122450686000_2021.11.08.21265884v1.21"
},
{
"DOI": "10.1016/j.ijid.2020.11.142",
"doi-asserted-by": "publisher",
"key": "2021111122450686000_2021.11.08.21265884v1.22"
},
{
"key": "2021111122450686000_2021.11.08.21265884v1.23",
"unstructured": "Yohei Doi , Masashi Kondo , Akifumi Matsuyama . Preliminary Report of the Favipiravir Observational Study in Japan. 2020;:6."
},
{
"DOI": "10.3389/fphar.2021.683296",
"doi-asserted-by": "publisher",
"key": "2021111122450686000_2021.11.08.21265884v1.24"
},
{
"DOI": "10.1124/dmd.111.043828",
"doi-asserted-by": "publisher",
"key": "2021111122450686000_2021.11.08.21265884v1.25"
},
{
"DOI": "10.1620/tjem.251.87",
"doi-asserted-by": "publisher",
"key": "2021111122450686000_2021.11.08.21265884v1.26"
}
],
"reference-count": 26,
"references-count": 26,
"relation": {
"has-review": [
{
"asserted-by": "object",
"id": "10.1162/2e3983f5.9e6920e5",
"id-type": "doi"
},
{
"asserted-by": "object",
"id": "10.1162/2e3983f5.947d3df2",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2021.11.08.21265884"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Favipiravir In Adults with Moderate to Severe COVID-19: A Phase 3 Multicentre, Randomized, Double-Blinded, Placebo-Controlled Trial",
"type": "posted-content"
}