Clinical characteristics and outcomes of hospitalized kidney transplant recipients with COVID-19 infection in China during the Omicron wave: a single-center cohort study
et al., Journal of Zhejiang University - SCIENCE B (Biomedicine & Biotechnology, doi:10.1631/jzus.B2300538, Jun 2024
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.0000000041 from 40 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
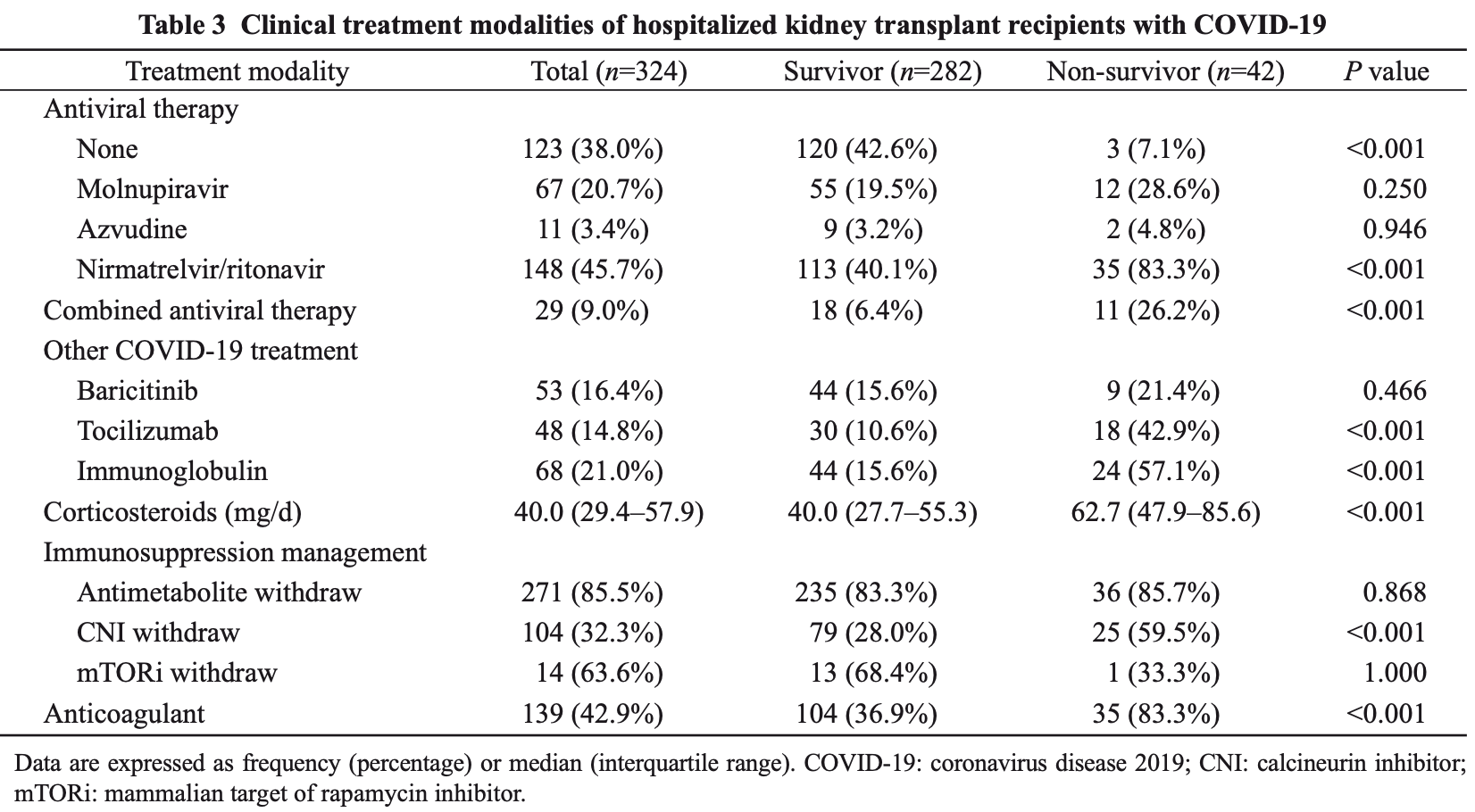
Retrospective 324 hospitalized kidney transplant recipients with COVID-19 showing no significant benefit with molnupiravir, paxlovid, or azvudine. The study was conducted during the omicron wave in China between December 2022 and January 2023. Adjusted results are only provided for all antivirals combined, however the results are similar before and after adjustment. Multivariable Cox regression analysis for all antivirals combined showed an adjusted hazard ratio for mortality of 6.06, p=0.099. While adjustment includes factors related to baseline severity, there may be residual confounding by indication.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments3.
Study covers molnupiravir, paxlovid, and azvudine.
|
risk of death, 42.3% higher, RR 1.42, p = 0.64, treatment 2 of 11 (18.2%), control 40 of 313 (12.8%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Xiong et al., Real-world data of Azvudine-induced hepatotoxicity among hospitalized COVID-19 patients in China: a retrospective case-control study, Frontiers in Pharmacology, doi:10.3389/fphar.2025.1558054.
Lv et al., 24 Jun 2024, retrospective, China, peer-reviewed, 10 authors, average treatment delay 14.0 days.
Clinical characteristics and outcomes of hospitalized kidney transplant recipients with COVID-19 infection in China during the Omicron wave: a single-center cohort study
doi:10.1631/jzus.B2300538
Background: Following the short-term outbreak of coronavirus disease 2019 in December 2022 in China, clinical data on kidney transplant recipients (KTRs) with COVID-19 are lacking. Methods: We conducted a single-center retrospective study to describe the clinical features, complications, and mortality rates of hospitalized KTRs infected with COVID-19 between Dec. 16, 2022 and Jan. 31, 2023. The patients were followed up until Mar. 31, 2023. Results: A total of 324 KTRs with COVID-19 were included. The median age was 49 years. The median time between the onset of symptoms and admission was 13 d. Molnupiravir, azvudine, and nirmatrelvir/ritonavir were administered to 67 (20.7%), 11 (3.4%), and 148 (45.7%) patients, respectively. Twenty-nine (9.0%) patients were treated with more than one antiviral agent. Forty-eight (14.8%) patients were treated with tocilizumab and 53 (16.4%) patients received baricitinib therapy. The acute kidney injury (AKI) occurred in 81 (25.0%) patients and 39 (12.0%) patients were admitted to intensive care units. Fungal infections were observed in 55 (17.0%) patients. Fifty (15.4%) patients lost their graft. The 28-d mortality rate of patients was 9.0% and 42 (13.0%) patients died by the end of follow-up. Multivariate Cox regression analysis identified that cerebrovascular disease, AKI incidence, interleukin (IL)-6 level of >6.8 pg/mL, daily dose of corticosteroids of >50 mg, and fungal infection were all associated with an increased risk of death for hospitalized patients. Conclusions: Our findings demonstrate that hospitalized KTRs with COVID-19 are at high risk of mortality. The administration of immunomodulators or the late application of antiviral drugs does not improve patient survival, while higher doses of corticosteroids may increase the death risk.
Author contributions Research idea and study design: Duo LV, Jianyong WU, and Jianghua CHEN; Data acquisition: Duo LV, Xishao XIE, Qinyun YANG, Zhimin CHEN, Guangjun LIU, Rending WANG, Wenhan PENG, and Hongfeng HUANG; Data analysis/ interpretation and statistical analysis: Duo LV and Xishao XIE; Supervision or mentorship: Jianyong WU and Jianghua CHEN. All the authors have read and approved the final manuscript, and therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines Duo LV, Xishao XIE, Qinyun YANG, Zhimin CHEN, Guangjun LIU, Wenhan PENG, Rending WANG, Hongfeng HUANG, Jianghua CHEN, and Jianyong WU declare that they have no conflicts of interest. This study was approved by the Clinical Research Ethics Committee of the First Affiliated Hospital of Zhejiang University (expedition review No. 63 in 2023) . The Ethics Committee authorized the informed consent waiver. This study was performed in accordance with the Declaration of Helsinki.
Supplementary information Table S1
References
Ahmad, Cisewski, Xu, COVID-19 mortality update -United States, 2022, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7218a4
Avery, Update on COVID-19 therapeutics for solid organ transplant recipients, including the omicron surge, Transplantation, doi:10.1097/TP.0000000000004200
Azzi, Bartash, Scalea, COVID-19 and solid organ transplantation: a review article, Transplantation, doi:10.1097/TP.0000000000003523
Berger, Hazzan, Kamar, Absence of mortality differences between the first and second COVID-19 waves in kidney transplant recipients, Kidney Int Rep, doi:10.1016/j.ekir.2022.09.007
Bernal, Gimeno, Alcaraz, Activating killercell immunoglobulin-like receptors are associated with the severity of coronavirus disease 2019, J Infect Dis, doi:10.1093/infdis/jiab228
Caillard, Anglicheau, Matignon, An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants, Kidney Int, doi:10.1016/j.kint.2020.08.005
Chan, Chaudhary, Saha, AKI in hospitalized patients with COVID-19, J Am Soc Nephrol, doi:10.1681/ASN.2020050615
Chaudhuri, Sasaki, Karkar, Corticosteroids in COVID-19 and non-COVID-19 ARDS: a systematic review and meta-analysis, Intensive Care Med, doi:10.1007/s00134-021-06394-2
Daniel, Sekulic, Kudose, Kidney allograft biopsy findings after COVID-19, Am J Transplant, doi:10.1111/ajt.16804
Daoud, Alqassieh, Alkhader, Immunosuppression in kidney transplant recipients with COVID-19 infection -where do we stand and where are we heading?, Ren Fail, doi:10.1080/0886022X.2021.1876730
Fang, Li, Yu, Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis, Aging, doi:10.18632/aging.103579
Farkash, Wilson, Jentzen, Ultrastructural evidence for direct renal infection with SARS-CoV-2, J Am Soc Nephrol, doi:10.1681/ASN.2020040432
Favà, Cucchiari, Montero, Clinical characteristics and risk factors for severe COVID-19 in hospitalized kidney transplant recipients: a multicentric cohort study, Am J Transplant, doi:10.1111/ajt.16246
Gangneux, Dannaoui, Fekkar, Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: the French multicentre MYCOVID study, Lancet Respir Med, doi:10.1016/S2213-2600(21)00442-2
Gupta, Wang, Hayek, Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19, JAMA Intern Med, doi:10.1001/jamainternmed.2020.6252
Gérard, Barbosa, Anglicheau, Association between maintenance immunosuppressive regimens and COVID-19 mortality in kidney transplant recipients, Transplantation, doi:10.1097/TP.0000000000004254
Hajibaratali, Amini, Dalili, Clinical outcomes of kidney recipients with COVID-19 (COVID-19 in kidney recipients), Transpl Immunol, doi:10.1016/j.trim.2022.101772
Hartzell, Bin, Benedetti, Evidence of potent humoral immune activity in COVID-19-infected kidney transplant recipients, Am J Transplant, doi:10.1111/ajt.16261
Jimeno, Ventura, Castellano, Prognostic implications of neutrophil-lymphocyte ratio in COVID-19, Eur J Clin Invest, doi:10.1111/eci.13404
Klopfenstein, Gendrin, Kadiane-Oussou, Tocilizumab in COVID-19 pneumonia: practical proposals based on a narrative review of randomised trials, Rev Med Virol, doi:10.1002/rmv.2239
Kremer, Pieters, Verhaar, A systematic review and meta-analysis of COVID-19 in kidney transplant recipients: lessons to be learned, Am J Transplant, doi:10.1111/ajt.16742
Kronbichler, Gauckler, Windpessl, COVID-19: implications for immunosuppression in kidney disease and transplantation, Nat Rev Nephrol, doi:10.1038/s41581-020-0305-6
Mahalingasivam, Su, Iwagami, COVID-19 and kidney disease: insights from epidemiology to inform clinical practice, Nat Rev Nephrol, doi:10.1038/s41581-022-00570-3
Mallhi, Khan, Alzarea, Incidence, risk factors and outcomes of acute kidney injury among COVID-19 patients: a systematic review of systematic reviews, Front Med, doi:10.3389/fmed.2022.973030
Masset, Gautier-Vargas, Cantarovich, Occurrence of de novo donor-specific antibodies after COVID-19 in kidney transplant recipients is low despite immunosuppression modulation, Kidney Int Rep, doi:10.1016/j.ekir.2022.01.1072
Mendoza, Ranganath, Chesdachai, Immunomodulators for severe coronavirus disease-2019 in transplant patients: do they increase the risk of secondary infection?, Transpl Infect Dis, doi:10.1111/tid.14050
Nab, Parker, Andrews, Changes in COVID-19-related mortality across key demographic and clinical subgroups in England from 2020 to 2022: a retrospective cohort study using the OpenSAFELY platform, Lancet Public Health, doi:10.1016/S2468-2667(23)00079-8
Nadim, Forni, Mehta, COVID-19associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup, Nat Rev Nephrol, doi:10.1038/s41581-020-00356-5
Ng, Hirsch, Hazzan, Outcomes among patients hospitalized with COVID-19 and acute kidney injury, Am J Kidney Dis, doi:10.1053/j.ajkd.2020.09.002
Nimmo, Gardiner, Ushiro-Lumb, The global impact of COVID-19 on solid organ transplantation: two years into a pandemic, Transplantation, doi:10.1097/TP.0000000000004151
Pereira, Aversa, Farr, Tocilizumab for severe COVID-19 in solid organ transplant recipients: a matched cohort study, Am J Transplant, doi:10.1111/ajt.16314
Pérez-Sáez, Blasco, Redondo-Pachón, Use of tocilizumab in kidney transplant recipients with COVID-19, Am J Transplant, doi:10.1111/ajt.16192
Richier, Jachiet, Bonnemains, Tocilizumab and COVID-19: timing of administration assessment, fect Dis Now, doi:10.1016/j.idnow.2021.06.304
Ronco, Reis, Syed, Management of acute kidney injury in patients with COVID-19, Lancet Respir Med, doi:10.1016/S2213-2600(20)30229-0
Saravolatz, Depcinski, Sharma, Molnupiravir and nirmatrelvir-ritonavir: oral coronavirus disease 2019 antiviral drugs, Clin Infect Dis, doi:10.1093/cid/ciac180
Singh, Oks, Husk, Impact of timing of tocilizumab use in hospitalized patients with SARS-CoV-2 infection, Respir Care, doi:10.4187/respcare.08779
Su, Yang, Wan, Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China, Kidney Int, doi:10.1016/j.kint.2020.04.003
Talic, Shah, Wild, Effectiveness of public health measures in reducing the incidence of covid-19, SARS-CoV-2 transmission, and covid-19 mortality: systematic review and meta-analysis, BMJ, doi:10.1136/bmj-2021-068302
Tan, Tan, Tan, Long-term kidney function recovery and mortality after COVID-19-associated acute kidney injury: an international multi-centre observational cohort study, eClinicalMedicine, doi:10.1016/j.eclinm.2022.101724
Tatum, Taghavi, Houghton, Neutrophil-tolymphocyte ratio and outcomes in Louisiana COVID-19 patients, Shock, doi:10.1097/SHK.0000000000001585
Tu, Mo, Zhang, Effects of different corticosteroid therapy on severe COVID-19 patients: a meta-analysis of randomized controlled trials, Expert Rev Respir Med, doi:10.1080/17476348.2021.1983429
Van Grootveld, Van Der Beek, Janssen, Incidence, risk factors and pre-emptive screening for COVID-19 associated pulmonary aspergillosis in an era of immunomodulant therapy, J Crit Care, doi:10.1016/j.jcrc.2023.154272
Wagner, Griesel, Mikolajewska, Systemic corticosteroids for the treatment of COVID-19, Cochrane Database Syst Rev, doi:10.1002/14651858
Wang, Koff, Hao, Effect of nirmatrelvir/ ritonavir on calcineurin inhibitor levels: early experience in four SARS-CoV-2 infected kidney transplant recipients, Am J Transplant, doi:10.1111/ajt.16997
Weiss, Hendrickx, Stensgaard, Kidney transplant and dialysis patients remain at increased risk for succumbing to COVID-19, Transplantation, doi:10.1097/TP.0000000000004462
Willicombe, Thomas, Mcadoo, COVID-19 and calcineurin inhibitors: should they get left out in the storm?, J Am Soc Nephrol, doi:10.1681/ASN.2020030348
Zhang, Han, Wu, COVID-19 in the immunocompromised population: data from renal allograft recipients throughout full cycle of the outbreak in Hubei province, China, Chin Med J, doi:10.1097/CM9.0000000000001538
Zhou, Kuang, Ma, Application of extracorporeal therapies in critically ill COVID-19 patients, J Zhejiang Univ-Sci B (Biomed & Biotechnol), doi:10.1631/jzus
Zhu, Gong, Liu, Coronavirus disease 2019 pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China, Eur Urol, doi:10.1016/j.eururo.2020.03.039
lv3
