Comparison of effectiveness and safety of molnupiravir versus sotrovimab for COVID‐19: A systematic review and meta‐analysis
et al., Immunity, Inflammation and Disease, doi:10.1002/iid3.1262, PROSPERO CRD42023429910, Apr 2024
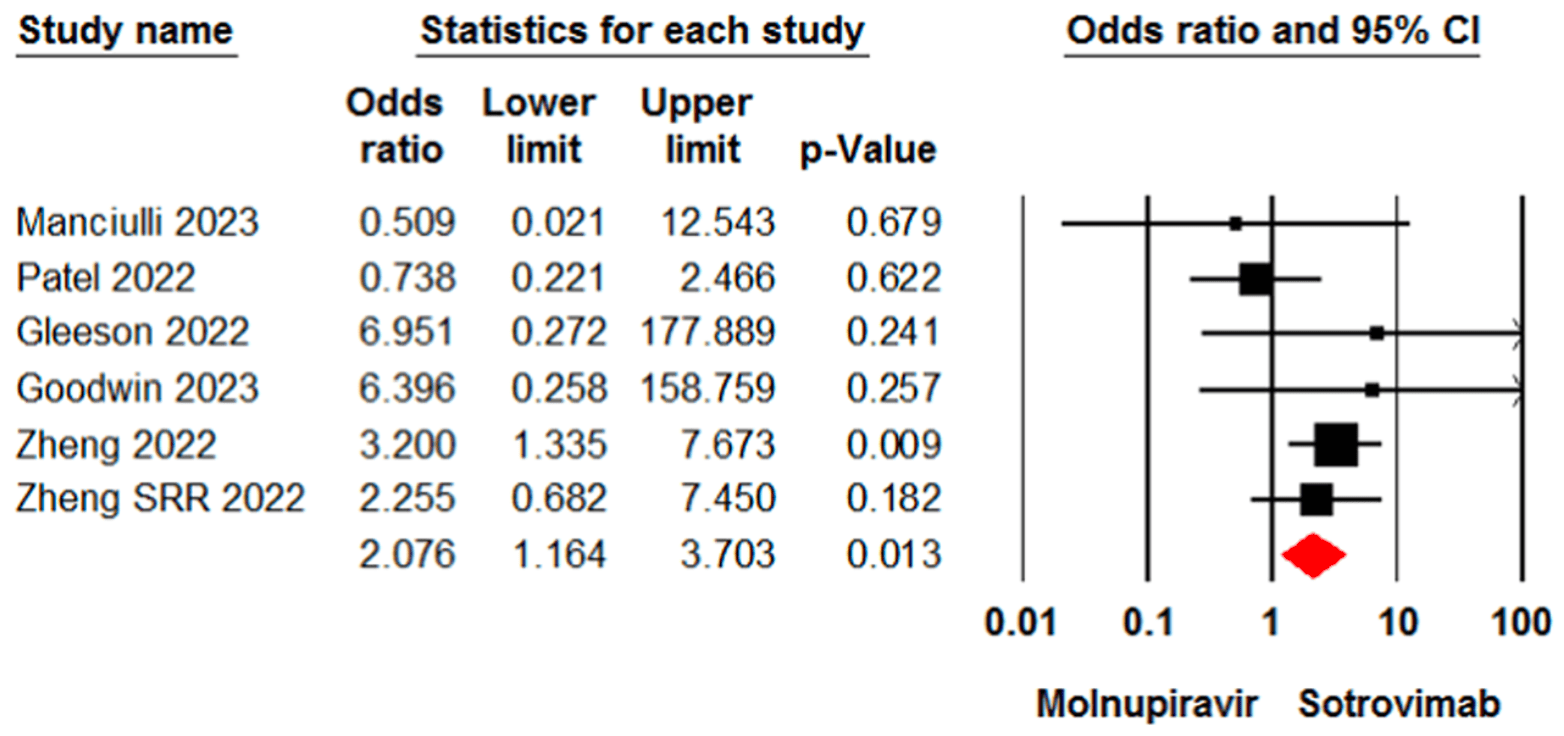
Meta analysis of 13 studies involving 16,166 patients showing higher mortality and higher incidence of adverse events with molnupiravir compared with sotrovimab.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
Currently there are 52 molnupiravir studies and meta-analysis shows:
| Outcome | Improvement |
|---|---|
| Mortality | 9% lower [-2‑19%] |
| Ventilation | 11% lower [-19‑34%] |
| ICU admission | 3% lower [-18‑20%] |
| Hospitalization | 0% lower [-7‑7%] |
| Cases | 24% fewer [-1‑43%] |
Study covers sotrovimab and molnupiravir.
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
Amani et al., 23 Apr 2024, peer-reviewed, 2 authors, trial PROSPERO CRD42023429910.
Contact: b_amani@alumnus.tums.ac.ir.
Comparison of effectiveness and safety of molnupiravir versus sotrovimab for COVID‐19: A systematic review and meta‐analysis
Immunity, Inflammation and Disease, doi:10.1002/iid3.1262
Background and Aim: This systematic review and meta-analysis aimed to compare the effectiveness and safety of molnupiravir and sotrovimab in the treatment of patients with coronavirus disease 2019 (COVID-19). Methods: Cochrane Library, Web of Science, PubMed, medRxiv, and Google Scholar were systematically searched to identify relevant evidence up to December 2023. The risk of bias was assessed using the risk of bias in nonrandomized studies of interventions tool. Data were analyzed using Comprehensive Meta-Analysis (CMA). Results: Our search identified and included 13 studies involving 16166 patients. The meta-analysis revealed a significant difference between the molnupiravir and sotrovimab groups in terms of the mortality rate (odds ratio [OR] = 2.07, 95% confidence interval [CI]: 1.16, 3.70). However, no significant difference was observed between the two groups in terms of hospitalization rate (OR = 0.71, 95% CI: 0.47, 1.06), death or hospitalization rate (OR = 1.51, 95% CI: 0.81, 2.83), and intensive care unit admission (OR = 0.59, 95% CI: 0.07, 4.84). In terms of safety, molnupiravir was associated with a higher incidence of adverse events (OR = 1.67, 95% CI: 1.21, 2.30).
Conclusion: The current findings indicate that sotrovimab may be more effective than molnupiravir in reducing the mortality rate in COVID-19 patients. However, no statistical difference was observed between the two treatments for other effectiveness outcomes. The certainty of evidence for these findings was rated as low or moderate. Further research is required to provide a better comparison of these interventions in treating COVID-19 patients.
AUTHOR CONTRIBUTIONS Conceptualization and project administration: Bahman Amani and Behnam Amani. Literature searching: Behnam Amani and Bahman Amani. Data extraction and quality assessment: Bahman Amani and Behnam Amani. Data analysis: Bahman Amani and Behnam Amani. Writing-original draft: Behnam Amani. Writing-review and editing: Bahman Amani and Behnam Amani.
CONFLICT OF INTEREST STATEMENT The authors declare no conflict of interest.
SUPPORTING INFORMATION Additional supporting information can be found online in the Supporting Information section at the end of this article.
References
Amani, Amani, Efficacy and safety of sotrovimab in patients with COVID-19: a rapid review and meta-analysis, Rev Med Virol
Amani, Zareei, Amani, Rapid review and meta-analysis of adverse events associated with molnupiravir in patients with COVID-19, Br J Clin Pharmacol
Behzad, Ali, Niinuma, Butler, Alqahtani, Real world effectiveness of sotrovimab in preventing COVID-19-related hospitalisation or death in patients infected with Omicron BA.2, J Infect Public Health
Benaicha, Khenhrani, Veer, Efficacy of molnupiravir for the treatment of mild or moderate COVID-19 in adults: a meta-analysis, Cureus
Caillard, Thaunat, COVID-19 vaccination in kidney transplant recipients, Nat Rev Nephrol
Calimeri, Lo Giudice, Buda, Role of the 1st booster dose of COVID-19 vaccine in the protection against the infection: a fundamental public health tool, J Prev Med Hyg
Cegolon, Pol, Simonetti, Filon, Luzzati, Molnupiravir, Nirmatrelvir/Ritonavir, or Sotrovimab for highrisk COVID-19 patients infected by the omicron variant: hospitalization, mortality, and time until negative swab test in real life, Pharmaceuticals
Cheng, Reyes, Satram, Real-world effectiveness of sotrovimab for the early treatment of COVID-19 during SARS-CoV-2 Delta and Omicron waves in the USA, Infect Dis Ther
Driouich, Bernadin, Touret, De Lamballerie, Nougairède, Activity of Sotrovimab against BQ.1.1 and XBB.1 Omicron sublineages in a hamster model, Antiviral Res
Drysdale, Tibble, Patel, Characteristics and outcomes of patients with COVID-19 at high risk of disease progression receiving sotrovimab, oral antivirals or no treatment in Scotland. medRxiv, doi:10.1101/2023.06.09.23291195
Evans, Qi, Adebayo, Real-world effectiveness of molnupiravir, nirmatrelvir-ritonavir, and sotrovimab on preventing hospital admission among higher-risk patients with COVID-19 in Wales: a retrospective cohort study, J Infect
Fernandes, Inchakalody, Merhi, Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines, Ann Med
Flisiak, Zarębska-Michaluk, Rogalska, Real-world experience with molnupiravir during the period of SARS-CoV-2 Omicron variant dominance, Pharmacol Rep
Gao, Liu, Li, Xu, Zhang et al., Molnupiravir for treatment of adults with mild or moderate COVID-19: a systematic review and meta-analysis of randomised controlled trials, Clin Microbiol Infect
Gleeson, Martin, Thomson, Kidney transplant recipients and omicron: outcomes, effect of vaccines and the efficacy and safety of novel treatments, medRxiv, doi:10.1101/2022.05.03.22274524
Goodwin, Thompson, Hall, Evaluation of outpatient treatment for non-hospitalised patients with COVID-19: the experience of a regional centre in the UK, PLoS One
Huang, Liu, Wu, Tsai, Lai, Clinical efficacy and safety of molnupiravir for nonhospitalized and hospitalized patients with COVID-19: a systematic review and metaanalysis of randomized control trials, J Med Virol
Hérate, Touret, Sotrovimab retains activity against SARS-CoV-2 Omicron variant BQ.1.1 in a non-human primate model, Heliyon
Kamal, Ramadan, Farraj, Bahig, Ezzat, The pill of recovery; Molnupiravir for treatment of COVID-19 patients; a systematic review, Saudi Pharm J, doi:10.1016/j.jsps.2022.03.002
Kauer, Totschnig, Karolyi, Zoufaly, Naegeli, Efficacy and tolerability of Sotrovimab Molnupiravir and Nirmatrelvir/Ritonavir for non-hospitalized patients at high risk for COVID-19: a retrospective, single-center analysis, doi:10.21203/rs.3.rs-2786240.v1
Kwok, Tsoi, Leung, Real-world study on effectiveness of molnupiravir and Nirmatrelvir-Ritonavir in unvaccinated patients with chronic respiratory diseases with confirmed SARS-CoV-2 infection managed in out-patient setting, Viruses
Lasagna, Cassaniti, Lilleri, Effectiveness of the available early therapies in reducing severe COVID-19 in nonhospitalized patients with solid tumors on active treatment, Front Med
Lin, Hung, Lai, Wang, Chen, The impact of neutralizing monoclonal antibodies on the outcomes of COVID-19 outpatients: a systematic review and metaanalysis of randomized controlled trials, J Med Virol
Maas, Strizki, Miller, Molnupiravir: mechanism of action, clinical, and translational science, Clin Transl Sci
Malin, Weibel, Gruell, Kreuzberger, Stegemann et al., Efficacy and safety of molnupiravir for the treatment of SARS-CoV-2 infection: a systematic review and meta-analysis, J Antimicrob Chemother
Manciulli, Spinicci, Rossetti, Safety and efficacy of outpatient treatments for COVID-19: real-life data from a regionwide cohort of high-risk patients in Tuscany, Italy (the FEDERATE Cohort), Viruses
Martin-Blondel, Marcelin, Soulié, Sotrovimab to prevent severe COVID-19 in high-risk patients infected with Omicron BA.2, J Infect
Mazzotta, Lepri, Colavita, Viral load decrease in SARS-CoV-2 BA.1 and BA.2 Omicron sublineages infection after treatment with monoclonal antibodies and direct antiviral agents, J Med Virol
Minotti, Mengato, Pieri, Early treatments of fragile children with COVID-19-results of CLEVER (Children COVID Early Treatment), a retrospective, observational study, Viruses
Miyashita, Nakamori, Ogata, Clinical efficacy of the neutralizing antibody therapy sotrovimab in patients with SARS-CoV-2 omicron BA.1 and BA.2 subvariant infections, Viruses
Nene, Santodomingo, Balog, Use of sotrovimab in vaccinated versus unvaccinated COVID-19 patients in a resource-limited emergency department during the omicron surge, J Am Coll Emerg Physicians Open
Page, Mckenzie, Bossuyt, The PRISMA 2020 statement: an updated guideline for reporting systematic reviews, Int J Surg
Painter, Holman, Bush, Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2, Antimicrob Agents Chemother, doi:10.1128/aac
Patel, Yarwood, Levick, Characteristics and outcomes of patients with COVID-19 at high-risk of disease progression receiving sotrovimab, oral antivirals or no treatment in England. medRxiv, doi:10.1101/2022.11.28.22282808
Radcliffe, Palacios, Azar, Cohen, Malinis, Realworld experience with available, outpatient COVID-19 therapies in solid organ transplant recipients during the omicron surge, Am J Transplant, doi:10.1111/ajt.17098
Ramos-Rincon, López-Carmona, Cobos-Palacios, Remdesivir in very old patients (≥ 80 years) hospitalized with COVID-19: real world data from the SEMI-COVID-19 registry, J Clin Med
Sterne, Hernán, Reeves, ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions, BMJ
Tian, Feng, Chen, Efficacy and safety of molnupiravir treatment for COVID-19: a systematic review and metaanalysis of randomized controlled trials, Int J Antimicro Agents
Tiseo, Barbieri, Galfo, Efficacy and safety of nirmatrelvir/ritonavir, molnupiravir, and remdesivir in a realworld cohort of outpatients with COVID-19 at high risk of progression: the PISA outpatient clinic experience, Infect Dis Ther
Touret, Baronti, Bouzidi, De Lamballerie, In vitro evaluation of therapeutic antibodies against a SARS-CoV-2 Omicron B.1.1.529 isolate, Sci Rep
Twohig, Nyberg, Zaidi, Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study, Lancet Infect Dis
Uraki, Kiso, Imai, Therapeutic efficacy of monoclonal antibodies and antivirals against SARS-CoV-2 Omicron BA.1 in Syrian hamsters, Nat Microbiol
Vito, Colpani, Poliseno, What is the efficacy of sotrovimab in reducing disease progression and death in people with COVID-19 during the omicron era? Answers from a real-life study, Viruses
Wen, Chen, Tang, Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis, Ann Med
Wong, Au, Lau, Lau, Cowling et al., Realworld effectiveness of early molnupiravir or nirmatrelvirritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study, Lancet Infect Dis
Zheng, Campbell, Carr, Comparative effectiveness of sotrovimab and molnupiravir for preventing severe COVID-19 outcomes in non-hospitalised patients on kidney replacement therapy: observational cohort study using the OpenSAFELY-UKRR linked platform and SRR database, medRxiv, doi:10.1101/2022.12.02.22283049
Zheng, Green, Tazare, Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe covid-19 outcomes in patients in the community: observational cohort study with the OpenSAFELY platform, BMJ, doi:10.1136/bmj-2022-071932
DOI record:
{
"DOI": "10.1002/iid3.1262",
"ISSN": [
"2050-4527",
"2050-4527"
],
"URL": "http://dx.doi.org/10.1002/iid3.1262",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Background and Aim</jats:title><jats:p>This systematic review and meta‐analysis aimed to compare the effectiveness and safety of molnupiravir and sotrovimab in the treatment of patients with coronavirus disease 2019 (COVID‐19).</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>Cochrane Library, Web of Science, PubMed, medRxiv, and Google Scholar were systematically searched to identify relevant evidence up to December 2023. The risk of bias was assessed using the risk of bias in nonrandomized studies of interventions tool. Data were analyzed using Comprehensive Meta‐Analysis (CMA).</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Our search identified and included 13 studies involving 16166 patients. The meta‐analysis revealed a significant difference between the molnupiravir and sotrovimab groups in terms of the mortality rate (odds ratio [OR] = 2.07, 95% confidence interval [CI]: 1.16, 3.70). However, no significant difference was observed between the two groups in terms of hospitalization rate (OR = 0.71, 95% CI: 0.47, 1.06), death or hospitalization rate (OR = 1.51, 95% CI: 0.81, 2.83), and intensive care unit admission (OR = 0.59, 95% CI: 0.07, 4.84). In terms of safety, molnupiravir was associated with a higher incidence of adverse events (OR = 1.67, 95% CI: 1.21, 2.30).</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>The current findings indicate that sotrovimab may be more effective than molnupiravir in reducing the mortality rate in COVID‐19 patients. However, no statistical difference was observed between the two treatments for other effectiveness outcomes. The certainty of evidence for these findings was rated as low or moderate. Further research is required to provide a better comparison of these interventions in treating COVID‐19 patients.</jats:p></jats:sec>",
"alternative-id": [
"10.1002/iid3.1262"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2023-09-04"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2024-04-12"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2024-04-23"
}
],
"author": [
{
"affiliation": [
{
"name": "Department of Health Management and Economics, School of Public Health Tehran University of Medical Sciences Tehran Iran"
}
],
"family": "Amani",
"given": "Bahman",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-4298-1807",
"affiliation": [
{
"name": "Department of Health Management and Economics, School of Public Health Tehran University of Medical Sciences Tehran Iran"
}
],
"authenticated-orcid": false,
"family": "Amani",
"given": "Behnam",
"sequence": "additional"
}
],
"container-title": "Immunity, Inflammation and Disease",
"container-title-short": "Immunity Inflam &amp; Disease",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2024,
4,
23
]
],
"date-time": "2024-04-23T13:44:49Z",
"timestamp": 1713879889000
},
"deposited": {
"date-parts": [
[
2024,
4,
23
]
],
"date-time": "2024-04-23T13:44:56Z",
"timestamp": 1713879896000
},
"indexed": {
"date-parts": [
[
2024,
4,
24
]
],
"date-time": "2024-04-24T00:36:39Z",
"timestamp": 1713918999165
},
"is-referenced-by-count": 0,
"issue": "4",
"issued": {
"date-parts": [
[
2024,
4
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2024,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 22,
"start": {
"date-parts": [
[
2024,
4,
23
]
],
"date-time": "2024-04-23T00:00:00Z",
"timestamp": 1713830400000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/iid3.1262",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2024,
4
]
]
},
"published-online": {
"date-parts": [
[
2024,
4,
23
]
]
},
"published-print": {
"date-parts": [
[
2024,
4
]
]
},
"publisher": "Wiley",
"reference": [
{
"article-title": "Role of the 1st booster dose of COVID‐19 vaccine in the protection against the infection: a fundamental public health tool",
"author": "Calimeri S",
"first-page": "520",
"issue": "4",
"journal-title": "J Prev Med Hyg",
"key": "e_1_2_9_2_1",
"volume": "63",
"year": "2022"
},
{
"DOI": "10.1038/s41581-021-00491-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_3_1"
},
{
"DOI": "10.1002/jmv.27623",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_4_1"
},
{
"DOI": "10.1080/07853890.2022.2034936",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_5_1"
},
{
"DOI": "10.1016/S1473-3099(22)00507-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_6_1"
},
{
"DOI": "10.3390/jcm11133769",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_7_1"
},
{
"DOI": "10.1101/2022.12.02.22283049",
"doi-asserted-by": "crossref",
"key": "e_1_2_9_8_1",
"unstructured": "ZhengB CampbellJ CarrEJ et al.Comparative effectiveness of sotrovimab and molnupiravir for preventing severe COVID‐19 outcomes in non‐hospitalised patients on kidney replacement therapy: observational cohort study using the OpenSAFELY‐UKRR linked platform and SRR database.medRxiv. Preprint posted online December 4 2022.doi:10.1101/2022.12.02.22283049"
},
{
"DOI": "10.3390/v15081757",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_9_1"
},
{
"DOI": "10.1016/j.jsps.2022.03.002",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_10_1"
},
{
"DOI": "10.1128/AAC.02428-20",
"article-title": "Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad‐spectrum oral antiviral agent with activity against SARS‐CoV‐2",
"author": "Painter WP",
"doi-asserted-by": "crossref",
"issue": "5",
"journal-title": "Antimicrob Agents Chemother",
"key": "e_1_2_9_11_1",
"volume": "65",
"year": "2021"
},
{
"DOI": "10.1007/s43440-022-00408-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_12_1"
},
{
"DOI": "10.3390/v15030610",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_13_1"
},
{
"DOI": "10.1007/s40121-022-00729-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_14_1"
},
{
"DOI": "10.1007/s40121-022-00755-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_15_1"
},
{
"DOI": "10.1002/emp2.12958",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_16_1"
},
{
"key": "e_1_2_9_17_1",
"unstructured": "Food and Drug Administration. Drug safety and availability.2022.https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-sotrovimab-emergency-use-authorization"
},
{
"DOI": "10.1016/j.jiph.2023.11.029",
"article-title": "Real world effectiveness of sotrovimab in preventing COVID‐19‐related hospitalisation or death in patients infected with Omicron BA.2",
"author": "Behzad A",
"doi-asserted-by": "crossref",
"first-page": "315",
"issue": "2",
"journal-title": "J Infect Public Health",
"key": "e_1_2_9_18_1",
"volume": "17",
"year": "2023"
},
{
"DOI": "10.1016/j.jinf.2022.06.033",
"article-title": "Sotrovimab to prevent severe COVID‐19 in high‐risk patients infected with Omicron BA.2",
"author": "Martin‐Blondel G",
"doi-asserted-by": "crossref",
"first-page": "e104",
"issue": "4",
"journal-title": "J Infect",
"key": "e_1_2_9_19_1",
"volume": "85",
"year": "2022"
},
{
"DOI": "10.3390/v15061300",
"article-title": "Clinical efficacy of the neutralizing antibody therapy sotrovimab in patients with SARS‐CoV‐2 omicron BA.1 and BA.2 subvariant infections",
"author": "Miyashita N",
"doi-asserted-by": "crossref",
"first-page": "1300",
"issue": "6",
"journal-title": "Viruses",
"key": "e_1_2_9_20_1",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1002/rmv.2402",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_21_1"
},
{
"DOI": "10.1002/jmv.28621",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_22_1"
},
{
"DOI": "10.1136/bmj-2022-071932",
"article-title": "Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe covid‐19 outcomes in patients in the community: observational cohort study with the OpenSAFELY platform",
"author": "Zheng B",
"doi-asserted-by": "crossref",
"journal-title": "BMJ",
"key": "e_1_2_9_23_1",
"volume": "379",
"year": "2022"
},
{
"DOI": "10.1101/2022.05.03.22274524",
"doi-asserted-by": "crossref",
"key": "e_1_2_9_24_1",
"unstructured": "GleesonS MartinP ThomsonT et al.Kidney transplant recipients and omicron: outcomes effect of vaccines and the efficacy and safety of novel treatments.medRxiv. Preprint posted online May 3 2022.doi:10.1101/2022.05.03.22274524"
},
{
"DOI": "10.3390/v15020438",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_25_1"
},
{
"DOI": "10.1111/cts.13732",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_26_1"
},
{
"DOI": "10.1016/S1473-3099(21)00475-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_27_1"
},
{
"DOI": "10.1016/j.ijsu.2021.105906",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_28_1"
},
{
"DOI": "10.1136/bmj.i4919",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_29_1"
},
{
"DOI": "10.3390/ph16050721",
"article-title": "Molnupiravir, Nirmatrelvir/Ritonavir, or Sotrovimab for high‐risk COVID‐19 patients infected by the omicron variant: hospitalization, mortality, and time until negative swab test in real life",
"author": "Cegolon L",
"doi-asserted-by": "crossref",
"first-page": "721",
"issue": "5",
"journal-title": "Pharmaceuticals",
"key": "e_1_2_9_30_1",
"volume": "16",
"year": "2023"
},
{
"DOI": "10.1101/2023.06.09.23291195",
"doi-asserted-by": "crossref",
"key": "e_1_2_9_31_1",
"unstructured": "DrysdaleM TibbleH PatelV et al.Characteristics and outcomes of patients with COVID‐19 at high risk of disease progression receiving sotrovimab oral antivirals or no treatment in Scotland.medRxiv. Preprint posted online June 9 2023.doi:10.1101/2023.06.09.23291195"
},
{
"DOI": "10.1016/j.jinf.2023.02.012",
"article-title": "Real‐world effectiveness of molnupiravir, nirmatrelvir‐ritonavir, and sotrovimab on preventing hospital admission among higher‐risk patients with COVID‐19 in Wales: a retrospective cohort study",
"author": "Evans A",
"doi-asserted-by": "crossref",
"first-page": "352",
"issue": "4",
"journal-title": "J Infect",
"key": "e_1_2_9_32_1",
"volume": "86",
"year": "2023"
},
{
"DOI": "10.1371/journal.pone.0281915",
"article-title": "Evaluation of outpatient treatment for non‐hospitalised patients with COVID‐19: the experience of a regional centre in the UK",
"author": "Goodwin AT",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "PLoS One",
"key": "e_1_2_9_33_1",
"volume": "18",
"year": "2023"
},
{
"DOI": "10.21203/rs.3.rs-2786240/v1",
"doi-asserted-by": "crossref",
"key": "e_1_2_9_34_1",
"unstructured": "KauerV TotschnigD AugustinM KarolyiM ZoufalyA NaegeliM.Efficacy and tolerability of Sotrovimab Molnupiravir and Nirmatrelvir/Ritonavir for non‐hospitalized patients at high risk for COVID‐19: a retrospective single‐center analysis. Preprint posted online April 10 2023.doi:10.21203/rs.3.rs-2786240.v1"
},
{
"DOI": "10.3389/fmed.2022.1036473",
"article-title": "Effectiveness of the available early therapies in reducing severe COVID‐19 in non‐hospitalized patients with solid tumors on active treatment",
"author": "Lasagna A",
"doi-asserted-by": "crossref",
"journal-title": "Front Med",
"key": "e_1_2_9_35_1",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1002/jmv.28186",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_36_1"
},
{
"DOI": "10.1101/2022.11.28.22282808",
"doi-asserted-by": "crossref",
"key": "e_1_2_9_37_1",
"unstructured": "PatelV YarwoodMJ LevickB et al. Characteristics and outcomes of patients with COVID‐19 at high‐risk of disease progression receiving sotrovimab oral antivirals or no treatment in England.medRxiv. Preprint posted online November 29 2022.doi:10.1101/2022.11.28.22282808"
},
{
"DOI": "10.1111/ajt.17098",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_38_1"
},
{
"DOI": "10.1080/07853890.2022.2031274",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_39_1"
},
{
"DOI": "10.1016/j.antiviral.2023.105638",
"article-title": "Activity of Sotrovimab against BQ.1.1 and XBB.1 Omicron sublineages in a hamster model",
"author": "Driouich J‐S",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral Res",
"key": "e_1_2_9_40_1",
"volume": "215",
"year": "2023"
},
{
"DOI": "10.1016/j.heliyon.2023.e16664",
"article-title": "Sotrovimab retains activity against SARS‐CoV‐2 Omicron variant BQ.1.1 in a non‐human primate model",
"author": "Hérate C",
"doi-asserted-by": "crossref",
"journal-title": "Heliyon",
"key": "e_1_2_9_41_1",
"volume": "9",
"year": "2023"
},
{
"DOI": "10.1038/s41598-022-08559-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_42_1"
},
{
"DOI": "10.1038/s41564-022-01170-4",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_43_1"
},
{
"DOI": "10.1093/jac/dkad132",
"article-title": "Efficacy and safety of molnupiravir for the treatment of SARS‐CoV‐2 infection: a systematic review and meta‐analysis",
"author": "Malin JJ",
"doi-asserted-by": "crossref",
"first-page": "1586",
"issue": "7",
"journal-title": "J Antimicrob Chemother",
"key": "e_1_2_9_44_1",
"volume": "78",
"year": "2023"
},
{
"DOI": "10.1016/j.ijantimicag.2023.106870",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_45_1"
},
{
"article-title": "Efficacy of molnupiravir for the treatment of mild or moderate COVID‐19 in adults: a meta‐analysis",
"author": "Benaicha K",
"issue": "5",
"journal-title": "Cureus",
"key": "e_1_2_9_46_1",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1016/j.cmi.2023.04.014",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_47_1"
},
{
"DOI": "10.3390/v15010192",
"article-title": "Early treatments of fragile children with COVID‐19—results of CLEVER (Children COVID Early Treatment), a retrospective, observational study",
"author": "Minotti C",
"doi-asserted-by": "crossref",
"first-page": "192",
"issue": "1",
"journal-title": "Viruses",
"key": "e_1_2_9_48_1",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1111/bcp.15449",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_49_1"
}
],
"reference-count": 48,
"references-count": 48,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/iid3.1262"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Comparison of effectiveness and safety of molnupiravir versus sotrovimab for COVID‐19: A systematic review and meta‐analysis",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "12"
}
amani3