Randomized double-blind clinical study in patients with COVID-19 to evaluate the safety and efficacy of a phytomedicine (P2Et)
et al., Frontiers in Medicine, doi:10.3389/fmed.2022.991873, NCT04410510, Sep 2022
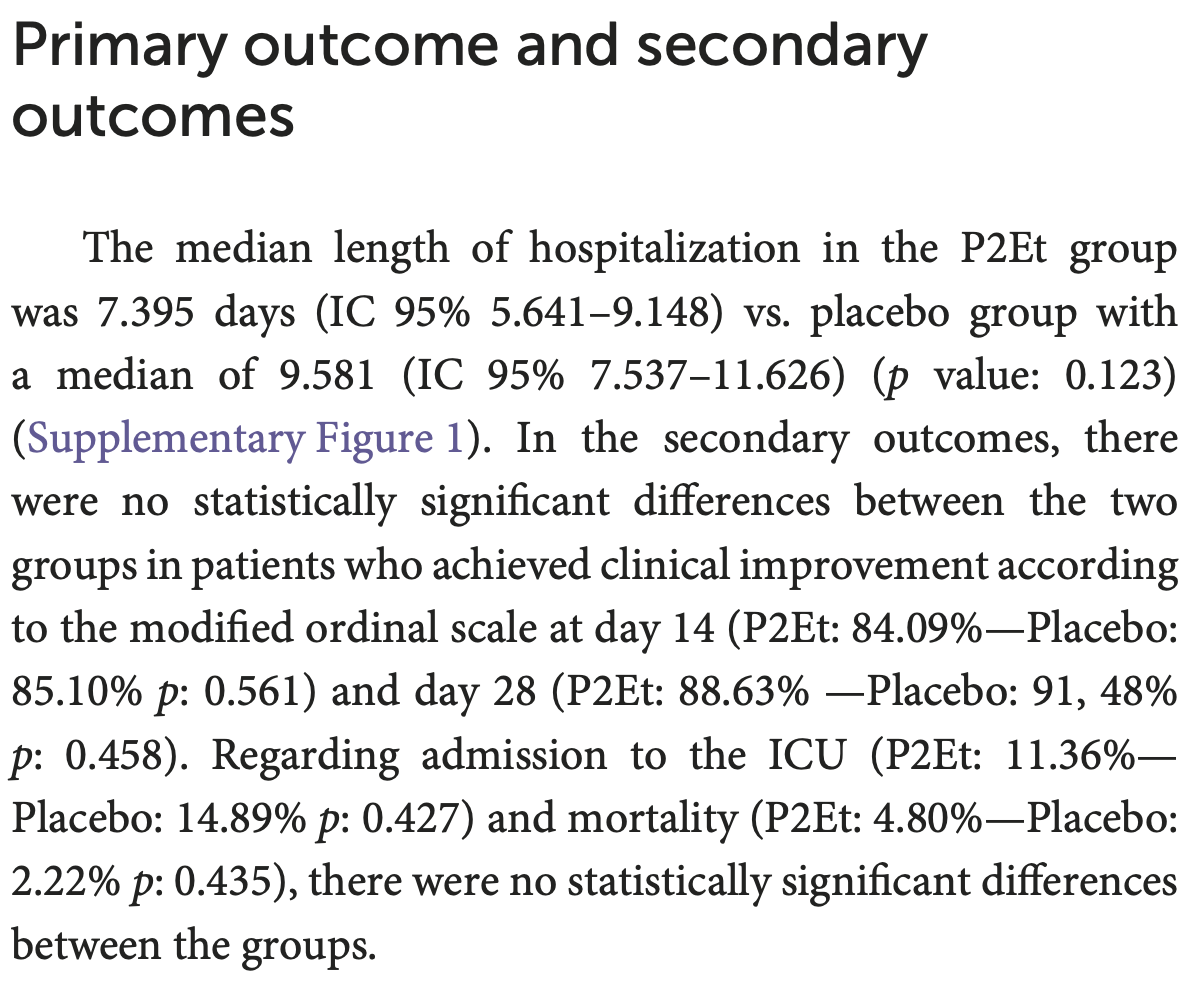
RCT 91 hospitalized COVID-19 patients in Colombia showing no significant differences in clinical outcomes with P2Et treatment. P2Et treatment was associated with reduced pro-inflammatory cytokines (G-CSF, IL-5, IL-18) and increased NK cells and other immune populations.
|
risk of death, 113.6% higher, RR 2.14, p = 0.61, treatment 2 of 44 (4.5%), control 1 of 47 (2.1%).
|
|
risk of ICU admission, 23.7% lower, RR 0.76, p = 0.76, treatment 5 of 44 (11.4%), control 7 of 47 (14.9%), NNT 28.
|
|
risk of no improvement, 33.5% higher, RR 1.34, p = 0.73, treatment 5 of 44 (11.4%), control 4 of 47 (8.5%), day 28.
|
|
risk of no improvement, 6.8% higher, RR 1.07, p = 1.00, treatment 7 of 44 (15.9%), control 7 of 47 (14.9%), day 14.
|
|
hospitalization time, 22.8% lower, relative time 0.77, p = 0.12, treatment 44, control 47.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Urueña et al., 8 Sep 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Colombia, peer-reviewed, 16 authors, study period 1 October, 2020 - 27 August, 2021, trial NCT04410510 (history).
P2Et is an oral phytomedicine, an extract from Caesalpinia spinosa, with potential anti-inflammatory and antiviral effects.
Randomized double-blind clinical study in patients with COVID-to evaluate the safety and efficacy of a phytomedicine (P Et)
Background: It has been proposed that polyphenols can be used in the development of new therapies against COVID-, given their ability to interfere with the adsorption and entrance processes of the virus, thus disrupting viral replication. Seeds from Caesalpinia spinosa, have been traditionally used for the treatment of inflammatory pathologies and respiratory diseases. Our team has obtained an extract called P Et, rich in polyphenols derived from gallic acid with significant antioxidant activity, and the ability to induce complete autophagy in tumor cells and reduce the systemic inflammatory response in animal models. Methods: In this work, a phase II multicenter randomized double-blind clinical trial on COVID-patients was designed to evaluate the impact of the P Et treatment on the clinical outcome and the immunological parameters related to the evolution of the disease. The Trial was registered with the number No. NCT * . A complementary study in an animal model of lung fibrosis was carried out to evaluate in situ lung changes after P Et in vivo administration. The ability of P Et to inhibit the viral load of murine and human coronaviruses in cellular models was also evaluated. Results: Patients treated with P Et were discharged on average after . days of admission vs. . days in the placebo group. Although a decrease in proinflammatory cytokines such as G-CSF, IL-, IL-, IL-, IP , MCP-, Frontiers in Medicine frontiersin.org Urueña et al. . /fmed. . MCP-and IL-was observed in both groups, P Et decreased to a greater extent G-CSF, IL-and IL-among others, which are related to lower recovery of patients in the long term. The frequency of T lymphocytes (LT) CD +, LT double negative (CD +CD -CD -), NK cells increased in the P Et group where the population of eosinophils was also significantly reduced. In the murine bleomycin model, P Et also reduced lung inflammation and fibrosis. P Et was able to reduce the viral replication of murine and human coronaviruses in vitro, showing its dual antiviral and anti-inflammatory role, key in disease control. Conclusions: Taken together these results suggest that P Et could be consider as a good co-adjuvant in the treatment of COVID-.
Ethics statement The studies involving human participants were reviewed and approved by the independent Ethics Committees (IECs) of the Hospital Universitario San Ignacio (Approval: 2020/050) and Centro Cardiovascular Colombiano Clínica Santa María (Approval: 2021/177). Trial was registered with the number No. NCT04410510 ( https://clinicaltrials.gov/ct2/ show/NCT04410510 ). The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by Veterinary Authority of the Swiss Canton Genève (authorization no. GE119/20).
Author contributions CU, RB-R, AB, and SF were responsible for the study design, interpretation of results, drafting and revising the manuscript. AG, CM, and PA participated in the recruitment of the COVID19 patients, acquisition, and analysis of clinical safety and efficacy data. KP, MZ-C, LF-A, WZ-B, and MR were responsible for the design, acquisition, and analysis of the in vitro COVID19 model. AG-C, HE-A, and CJ were responsible of the design, acquisition, and analysis of the in vivo lung model. All authors participated in drafting and revising the manuscript and have read and approved the final manuscript.
Conflict of interest Authors SF and CU are inventors of a granted patent related to P2Et. Authors SF, CU, and RB-R are partners of the DreemBio company who was a licensee of related patents. The remaining authors declare that the research was conducted in the absence of any..
References
Administration, Botanical Drug Development Guidance for Industry
Aruoma, Spencer, Rossi, Aeschbach, Khan et al., An evaluation of the antioxidant and antiviral action of extracts of rosemary and Provencal herbs, Food Chem Toxicol, doi:10.1016/0278-6915(96)00004-X
Ballesteros-Ramírez, Durán, Fiorentino, Genotoxicity and mutagenicity assessment of a standardized extract (P2Et) obtained from Caesalpinia spinosa, Toxicol Rep, doi:10.1016/j.toxrep.2020.12.024
Brendler, Al-Harrasi, Bauer, Gafner, Hardy et al., Botanical drugs and supplements affecting the immune response in the time of COVID-19: implications for research and clinical practice, Phyther Res, doi:10.1002/ptr.7008
Castañeda, Pombo, Urueña, Hernandez, Fiorentino, gallotannin-rich fraction from Caesalpinia spinosa (Molina) Kuntze displays cytotoxic activity and raises sensitivity to doxorubicin in a leukemia cell line, BMC Complement Altern Med, doi:10.1186/1472-6882-12-38
Choi, Moon, Yang, Kim, Understanding viral infection mechanisms and patient symptoms for the development of COVID-19 therapeutics, Int J Mol Sci, doi:10.3390/ijms22041737
Da Silva Antonio, Wiedemann, Vf, Natural products' role against COVID-19, RSC Adv, doi:10.1039/D0RA03774E
Duran, Ballesteros-Ramírez, Tellez, Torregrosa, Olejua et al., Safety evaluation in healthy colombian volunteers of P2Et extract obtained from caesalpinia spinosa: design 3+ 3 phase i clinical trial, Evidence-Based Complement Altern Med, doi:10.1155/2022/7943001
Dutra, Campos, Santos, Calixto, Medicinal plants in Brazil: Pharmacological studies, drug discovery, challenges and perspectives, Pharmacol Res, doi:10.1016/j.phrs.2016.01.021
Gounni, Nutku, Koussih, Aris, Louahed et al., IL-9 expression by human eosinophils: regulation by IL-1β and TNF-α, J Allergy Clin Immunol, doi:10.1067/mai.2000.109172
Guo, Kong, Meydani, Dietary polyphenols, inflammation, and cancer, Nutr Cancer, doi:10.1080/01635580903285098
Huang, Li, Luo, Wu, Su et al., The inflammatory factors associated with disease severity to predict COVID-19 progression, J Immunol, doi:10.4049/jimmunol.2001327
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Jain, Iyengar, Ish, Elucidating causes of COVID-19 infection and related deaths after vaccination, Diabetes Metab Syndr Clin Res Rev, doi:10.1016/j.dsx.2021.102212
Kawai, Tsuno, Kitayama, Sunami, Takahashi et al., Catechin inhibits adhesion and migration of peripheral blood B cells by blocking CD11b, Immunopharmacol Immunotoxicol, doi:10.3109/08923973.2010.522195
Kouro, Takatsu, IL-5-and eosinophil-mediated inflammation: from discovery to therapy, Int Immunol, doi:10.1093/intimm/dxp102
Levy, Delvin, Marcil, Spahis, Can phytotherapy with polyphenols serve as a powerful approach for the prevention and therapy tool of novel coronavirus disease 2019 (COVID-19)?, Am J Physiol Metab, doi:10.1152/ajpendo.00298.2020
Liao, Liu, Yuan, Wen, Xu et al., Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19, Nat Med, doi:10.1038/s41591-020-0901-9
Lim, Ng, Tam, Liu, Human coronaviruses: a review of virus-host interactions, Diseases, doi:10.3390/diseases4030026
Limanaqi, Busceti, Biagioni, Lazzeri, Forte et al., Cell clearing systems as targets of polyphenols in viral infections: potential implications for COVID-19 pathogenesis, Antioxidants, doi:10.3390/antiox9111105
Long, Liao, Wang, Lu, player and coordinator: the versatile roles of eosinophils in the immune system, Transfus Med Hemotherapy, doi:10.1159/000445215
Marín-Palma, Tabares-Guevara, Zapata-Cardona, Flórez-Álvarez, Yepes et al., Curcumin Inhibits invitro SARS-CoV-2 infection in vero E6 cells through multiple antiviral mechanisms, Molecules, doi:10.3390/molecules26226900
Muhammad, Kani, Iliya, Muhammad, Binji et al., Deficiency of antioxidants and increased oxidative stress in COVID-19 patients: a cross-sectional comparative study in Jigawa, Northwestern Nigeria, SAGE Open Med, doi:10.1177/2050312121991246
Nadeem, Chhabra, Masood, Raj, Increased oxidative stress and altered levels of antioxidants in asthma, J Allergy Clin Immunol, doi:10.1067/mai.2003.17
Neeland, Bannister, Clifford, Dohle, Mulholland et al., Innate cell profiles during the acute and convalescent phase of SARS-CoV-2 infection in children, Nat Commun, doi:10.1038/s41467-021-21414-x
Neyt, Geurtsvankessel, Lambrecht, Double-negative T resident memory cells of the lung react to influenza virus infection via CD11chi dendritic cells, Mucosal Immunol, doi:10.1038/mi.2015.91
Olson, Davis, Diagnosis and treatment of adults with communityacquired pneumonia, JAMA, doi:10.1001/jama.2019.21118
Prieto, Cao, Trillo-Tinoco, Sierra, Urueña, Polyphenol-rich extract induces apoptosis with immunogenic markers in melanoma cells through the ER stress-associated kinase PERK, Cell death Discov, doi:10.1038/s41420-019-0214-2
Rubenfeld, Thompson, Ferguson, Camporota, Acute respiratory distress syndrome, The Berlin Def JAMA, doi:10.1001/jama.2012.5669
Sandoval, Urueña, Llano, Gómez-Cadena, Hernández et al., Standardized extract from Caesalpinia spinosa is cytotoxic over cancer stem cells and enhance anticancer activity of doxorubicin, Am J Chin Med, doi:10.1142/S0192415X16500956
Singer, Deutschman, Seymour, Shankar-Hari, Annane et al., The third international consensus definitions for sepsis and septic shock (Sepsis-3), JAMA, doi:10.1001/jama.2016.0287
Smadja, Mentzer, Fontenay, Laffan, Ackermann et al., COVID-19 is a systemic vascular hemopathy: insight for mechanistic and clinical aspects, Angiogenesis, doi:10.1007/s10456-021-09805-6
Stebbing, Phelan, Griffin, Tucker, Oechsle et al., COVID-19: combining antiviral and anti-inflammatory treatments, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30132-8
Taylor, Huang, Mallett, Stathopoulou, Felizardo et al., PD-1 regulates KLRG1+ group 2 innate lymphoid cells, J Exp Med, doi:10.1084/jem.20161653
Trujillo, Consenso colombiano de atención, diagnóstico y manejo de la infección por SARS-CoV-2/COVID-19 en establecimientos de atención de la salud. Recomendaciones basadas en consenso de expertos e informadas en la evidencia, Infectio, doi:10.22354/in.v24i3.852
Udwadia, Koul, Richeldi, Post-COVID lung fibrosis: the tsunami that will follow the earthquake, Lung India Off Organ Indian Chest Soc, doi:10.4103/lungindia.lungindia_818_20
Urueña, Mancipe, Hernandez, Castañeda, Pombo et al., Gallotannin-rich Caesalpinia spinosa fraction decreases the primary tumor and factors associated with poor prognosis in a murine breast cancer model, BMC Complement Altern Med, doi:10.1186/1472-6882-13-74
Van Daal, Folkerts, Garssen, Braber, Pharmacological modulation of immune responses by nutritional components, Pharmacol Rev, doi:10.1124/pharmrev.120.000063
Van Dongen, Lhermitte, Böttcher, Almeida, Van Der Velden et al., EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes, Leukemia, doi:10.1038/leu.2012.120
Wu, Zheng, Sheng, Han, Yang et al., CD3+ CD4-CD8-(Double-Negative) T Cells in Inflammation, Immune Disorders and Cancer, Front Immunol, doi:10.3389/fimmu.2022.816005
Zahran, Zahran, Mady, Mahran, Makboul, Differential alterations in peripheral lymphocyte subsets in COVID-19 patients: upregulation of double-positive and double-negative T cells, Multidiscip Respir Med, doi:10.4081/mrm.2021.758
Zapata-Cardona, Flórez-Álvarez, Zapata-Builes, Guerra-Sandoval, Guerra-Almonacid et al., Atorvastatin Effectively Inhibits Ancestral and Two Emerging Variants of SARS-CoV-2 in vitro, Front Microbiol, doi:10.3389/fmicb.2022.721103
DOI record:
{
"DOI": "10.3389/fmed.2022.991873",
"ISSN": [
"2296-858X"
],
"URL": "http://dx.doi.org/10.3389/fmed.2022.991873",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p>It has been proposed that polyphenols can be used in the development of new therapies against COVID-19, given their ability to interfere with the adsorption and entrance processes of the virus, thus disrupting viral replication. Seeds from <jats:italic>Caesalpinia spinosa</jats:italic>, have been traditionally used for the treatment of inflammatory pathologies and respiratory diseases. Our team has obtained an extract called P2Et, rich in polyphenols derived from gallic acid with significant antioxidant activity, and the ability to induce complete autophagy in tumor cells and reduce the systemic inflammatory response in animal models.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>In this work, a phase II multicenter randomized double-blind clinical trial on COVID-19 patients was designed to evaluate the impact of the P2Et treatment on the clinical outcome and the immunological parameters related to the evolution of the disease. The Trial was registered with the number No. NCT04410510<jats:sup>*</jats:sup>. A complementary study in an animal model of lung fibrosis was carried out to evaluate <jats:italic>in situ</jats:italic> lung changes after P2Et <jats:italic>in vivo</jats:italic> administration. The ability of P2Et to inhibit the viral load of murine and human coronaviruses in cellular models was also evaluated.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Patients treated with P2Et were discharged on average after 7.4 days of admission vs. 9.6 days in the placebo group. Although a decrease in proinflammatory cytokines such as G-CSF, IL-15, IL-12, IL-6, IP10, MCP-1, MCP-2 and IL-18 was observed in both groups, P2Et decreased to a greater extent G-CSF, IL-6 and IL-18 among others, which are related to lower recovery of patients in the long term. The frequency of T lymphocytes (LT) CD3+, LT double negative (CD3+CD4-CD8-), NK cells increased in the P2Et group where the population of eosinophils was also significantly reduced. In the murine bleomycin model, P2Et also reduced lung inflammation and fibrosis. P2Et was able to reduce the viral replication of murine and human coronaviruses <jats:italic>in vitro</jats:italic>, showing its dual antiviral and anti-inflammatory role, key in disease control.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>Taken together these results suggest that P2Et could be consider as a good co-adjuvant in the treatment of COVID-19.</jats:p></jats:sec><jats:sec><jats:title>Clinical trail registration</jats:title><jats:p><jats:ext-link>https://clinicaltrials.gov/ct2/show/NCT04410510</jats:ext-link>, identifier: NCT04410510.</jats:p></jats:sec>",
"alternative-id": [
"10.3389/fmed.2022.991873"
],
"article-number": "991873",
"author": [
{
"affiliation": [],
"family": "Urueña",
"given": "Claudia",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ballesteros-Ramírez",
"given": "Ricardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gomez-Cadena",
"given": "Alejandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barreto",
"given": "Alfonso",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Prieto",
"given": "Karol",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Quijano",
"given": "Sandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aschner",
"given": "Pablo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martínez",
"given": "Carlos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zapata-Cardona",
"given": "Maria I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "El-Ahanidi",
"given": "Hajar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jandus",
"given": "Camilla",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Florez-Alvarez",
"given": "Lizdany",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rugeles",
"given": "Maria Teresa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zapata-Builes",
"given": "Wildeman",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garcia",
"given": "Angel Alberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fiorentino",
"given": "Susana",
"sequence": "additional"
}
],
"container-title": "Frontiers in Medicine",
"container-title-short": "Front. Med.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2022,
9,
8
]
],
"date-time": "2022-09-08T11:29:27Z",
"timestamp": 1662636567000
},
"deposited": {
"date-parts": [
[
2022,
9,
8
]
],
"date-time": "2022-09-08T11:29:38Z",
"timestamp": 1662636578000
},
"indexed": {
"date-parts": [
[
2025,
11,
14
]
],
"date-time": "2025-11-14T16:56:12Z",
"timestamp": 1763139372662,
"version": "3.41.2"
},
"is-referenced-by-count": 10,
"issued": {
"date-parts": [
[
2022,
9,
8
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
8
]
],
"date-time": "2022-09-08T00:00:00Z",
"timestamp": 1662595200000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2022.991873/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2022,
9,
8
]
]
},
"published-online": {
"date-parts": [
[
2022,
9,
8
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1016/j.phrs.2016.01.021",
"article-title": "Medicinal plants in Brazil: Pharmacological studies, drug discovery, challenges and perspectives",
"author": "Dutra",
"doi-asserted-by": "publisher",
"first-page": "4",
"journal-title": "Pharmacol Res.",
"key": "B1",
"volume": "112",
"year": "2016"
},
{
"DOI": "10.1002/ptr.7008",
"article-title": "Botanical drugs and supplements affecting the immune response in the time of COVID-19: implications for research and clinical practice",
"author": "Brendler",
"doi-asserted-by": "publisher",
"first-page": "3013",
"journal-title": "Phyther Res.",
"key": "B2",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1067/mai.2003.17",
"article-title": "Increased oxidative stress and altered levels of antioxidants in asthma",
"author": "Nadeem",
"doi-asserted-by": "publisher",
"first-page": "72",
"journal-title": "J Allergy Clin Immunol.",
"key": "B3",
"volume": "111",
"year": "2003"
},
{
"DOI": "10.1016/0278-6915(96)00004-X",
"article-title": "An evaluation of the antioxidant and antiviral action of extracts of rosemary and Provencal herbs",
"author": "Aruoma",
"doi-asserted-by": "publisher",
"first-page": "449",
"journal-title": "Food Chem Toxicol.",
"key": "B4",
"volume": "34",
"year": "1996"
},
{
"DOI": "10.1177/2050312121991246",
"article-title": "Deficiency of antioxidants and increased oxidative stress in COVID-19 patients: a cross-sectional comparative study in Jigawa, Northwestern Nigeria",
"author": "Muhammad",
"doi-asserted-by": "publisher",
"first-page": "2050312121991246",
"journal-title": "SAGE Open Med.",
"key": "B5",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1039/D0RA03774E",
"article-title": "Natural products' role against COVID-19",
"author": "da Silva Antonio",
"doi-asserted-by": "publisher",
"first-page": "23379",
"journal-title": "RSC Adv.",
"key": "B6",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China",
"author": "Huang",
"doi-asserted-by": "publisher",
"first-page": "497",
"journal-title": "Lancet.",
"key": "B7",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(20)30132-8",
"article-title": "COVID-19: combining antiviral and anti-inflammatory treatments",
"author": "Stebbing",
"doi-asserted-by": "publisher",
"first-page": "400",
"journal-title": "Lancet Infect Dis.",
"key": "B8",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1080/01635580903285098",
"article-title": "Dietary polyphenols, inflammation, and cancer",
"author": "Guo",
"doi-asserted-by": "publisher",
"first-page": "807",
"journal-title": "Nutr Cancer.",
"key": "B9",
"volume": "61",
"year": "2009"
},
{
"DOI": "10.1038/s41420-019-0214-2",
"article-title": "Polyphenol-rich extract induces apoptosis with immunogenic markers in melanoma cells through the ER stress-associated kinase PERK",
"author": "Prieto",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Cell death Discov.",
"key": "B10",
"volume": "5",
"year": "2019"
},
{
"DOI": "10.1142/S0192415X16500956",
"article-title": "Standardized extract from Caesalpinia spinosa is cytotoxic over cancer stem cells and enhance anticancer activity of doxorubicin",
"author": "Sandoval",
"doi-asserted-by": "publisher",
"first-page": "1693",
"journal-title": "Am J Chin Med.",
"key": "B11",
"volume": "44",
"year": "2016"
},
{
"DOI": "10.3390/antiox9111105",
"article-title": "Cell clearing systems as targets of polyphenols in viral infections: potential implications for COVID-19 pathogenesis",
"author": "Limanaqi",
"doi-asserted-by": "publisher",
"first-page": "1105",
"journal-title": "Antioxidants.",
"key": "B12",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1186/1472-6882-13-74",
"article-title": "Gallotannin-rich Caesalpinia spinosa fraction decreases the primary tumor and factors associated with poor prognosis in a murine breast cancer model",
"author": "Urueña",
"doi-asserted-by": "publisher",
"first-page": "74",
"journal-title": "BMC Complement Altern Med.",
"key": "B13",
"volume": "13",
"year": "2013"
},
{
"article-title": "Buenas prácticas clínicas: documento de las Américas",
"key": "B14",
"volume-title": "IV Conferencia Panamericana para la Armonización de la Reglamentación Farmacéutica",
"year": "2005"
},
{
"DOI": "10.1001/jama.2013.281053",
"article-title": "World medical association declaration of helsinki: ethical principles for medical research involving human subjects",
"doi-asserted-by": "publisher",
"first-page": "2191",
"journal-title": "JAMA",
"key": "B15",
"volume": "310",
"year": "2013"
},
{
"DOI": "10.1001/jama.2012.5669",
"article-title": "Acute respiratory distress syndrome",
"author": "Rubenfeld",
"doi-asserted-by": "publisher",
"first-page": "2526",
"journal-title": "The Berlin Def JAMA.",
"key": "B16",
"volume": "307",
"year": "2012"
},
{
"DOI": "10.1001/jama.2019.21118",
"article-title": "Diagnosis and treatment of adults with community-acquired pneumonia",
"author": "Olson",
"doi-asserted-by": "publisher",
"first-page": "885",
"journal-title": "JAMA.",
"key": "B17",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1001/jama.2016.0287",
"article-title": "The third international consensus definitions for sepsis and septic shock (Sepsis-3)",
"author": "Singer",
"doi-asserted-by": "publisher",
"first-page": "801",
"journal-title": "JAMA.",
"key": "B18",
"volume": "315",
"year": "2016"
},
{
"DOI": "10.22354/in.v24i3.852",
"article-title": "Consenso colombiano de atención, diagnóstico y manejo de la infección por SARS-CoV-2/COVID-19 en establecimientos de atención de la salud. Recomendaciones basadas en consenso de expertos e informadas en la evidencia",
"author": "Saavedra Trujillo",
"doi-asserted-by": "publisher",
"first-page": "186",
"journal-title": "Infectio.",
"key": "B19",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1016/j.toxrep.2020.12.024",
"article-title": "Genotoxicity and mutagenicity assessment of a standardized extract (P2Et) obtained from Caesalpinia spinosa",
"author": "Ballesteros-Ramírez",
"doi-asserted-by": "publisher",
"first-page": "258",
"journal-title": "Toxicol Rep.",
"key": "B20",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1186/1472-6882-12-38",
"article-title": "A gallotannin-rich fraction from Caesalpinia spinosa (Molina) Kuntze displays cytotoxic activity and raises sensitivity to doxorubicin in a leukemia cell line",
"author": "Castañeda",
"doi-asserted-by": "publisher",
"first-page": "38",
"journal-title": "BMC Complement Altern Med.",
"key": "B21",
"volume": "12",
"year": "2012"
},
{
"key": "B22",
"volume-title": "Botanical Drug Development Guidance for Industry",
"year": "2016"
},
{
"article-title": "M3 (R2): Nonclinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals",
"key": "B23",
"volume-title": "International conference on harmonization of technical requirements for the registration of pharmaceuticals for human use",
"year": "2009"
},
{
"article-title": "Safety evaluation in healthy colombian volunteers of P2Et extract obtained from caesalpinia spinosa: design 3+ 3 phase i clinical trial",
"author": "Duran",
"first-page": "1",
"key": "B24",
"volume-title": "Evidence-Based Complement Altern Med",
"year": "2022"
},
{
"key": "B25",
"volume-title": "National Institutes of Health Common Terminology Criteria for Adverse Events (CTCAE)",
"year": "2009"
},
{
"DOI": "10.3389/fmicb.2022.721103",
"article-title": "Atorvastatin Effectively Inhibits Ancestral and Two Emerging Variants of SARS-CoV-2 in vitro",
"author": "Zapata-Cardona",
"doi-asserted-by": "publisher",
"first-page": "721103",
"journal-title": "Front Microbiol.",
"key": "B26",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.3390/molecules26226900",
"article-title": "Curcumin Inhibits invitro SARS-CoV-2 infection in vero E6 cells through multiple antiviral mechanisms",
"author": "Marín-Palma",
"doi-asserted-by": "publisher",
"first-page": "6900",
"journal-title": "Molecules.",
"key": "B27",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.1038/leu.2012.120",
"article-title": "EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes",
"author": "Van Dongen",
"doi-asserted-by": "publisher",
"first-page": "1908",
"journal-title": "Leukemia.",
"key": "B28",
"volume": "26",
"year": "2012"
},
{
"DOI": "10.1007/s10456-021-09805-6",
"article-title": "COVID-19 is a systemic vascular hemopathy: insight for mechanistic and clinical aspects",
"author": "Smadja",
"doi-asserted-by": "publisher",
"first-page": "755",
"journal-title": "Angiogenesis.",
"key": "B29",
"volume": "24",
"year": "2021"
},
{
"DOI": "10.3390/ijms22041737",
"article-title": "Understanding viral infection mechanisms and patient symptoms for the development of COVID-19 therapeutics",
"author": "Choi",
"doi-asserted-by": "publisher",
"first-page": "1737",
"journal-title": "Int J Mol Sci.",
"key": "B30",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1016/j.dsx.2021.102212",
"article-title": "Elucidating causes of COVID-19 infection and related deaths after vaccination",
"author": "Jain",
"doi-asserted-by": "publisher",
"first-page": "102212",
"journal-title": "Diabetes Metab Syndr Clin Res Rev.",
"key": "B31",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1152/ajpendo.00298.2020",
"article-title": "Can phytotherapy with polyphenols serve as a powerful approach for the prevention and therapy tool of novel coronavirus disease 2019 (COVID-19)?",
"author": "Levy",
"doi-asserted-by": "publisher",
"first-page": "E689",
"journal-title": "Am J Physiol Metab.",
"key": "B32",
"volume": "319",
"year": "2020"
},
{
"DOI": "10.1038/s41467-021-21414-x",
"article-title": "Innate cell profiles during the acute and convalescent phase of SARS-CoV-2 infection in children",
"author": "Neeland",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Nat Commun.",
"key": "B33",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1038/s41591-020-0901-9",
"article-title": "Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19",
"author": "Liao",
"doi-asserted-by": "publisher",
"first-page": "842",
"journal-title": "Nat Med.",
"key": "B34",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.4049/jimmunol.2001327",
"article-title": "The inflammatory factors associated with disease severity to predict COVID-19 progression",
"author": "Huang",
"doi-asserted-by": "publisher",
"first-page": "1597",
"journal-title": "J Immunol.",
"key": "B35",
"volume": "206",
"year": "2021"
},
{
"DOI": "10.3390/diseases4030026",
"article-title": "Human coronaviruses: a review of virus–host interactions",
"author": "Lim",
"doi-asserted-by": "publisher",
"first-page": "26",
"journal-title": "Diseases.",
"key": "B36",
"volume": "4",
"year": "2016"
},
{
"DOI": "10.4103/lungindia.lungindia_818_20",
"article-title": "Post-COVID lung fibrosis: the tsunami that will follow the earthquake",
"author": "Udwadia",
"doi-asserted-by": "publisher",
"first-page": "S41",
"journal-title": "Lung India Off Organ Indian Chest Soc.",
"key": "B37",
"volume": "38",
"year": "2021"
},
{
"DOI": "10.1084/jem.20161653",
"article-title": "PD-1 regulates KLRG1+ group 2 innate lymphoid cells",
"author": "Taylor",
"doi-asserted-by": "publisher",
"first-page": "1663",
"journal-title": "J Exp Med.",
"key": "B38",
"volume": "214",
"year": "2017"
},
{
"DOI": "10.1093/intimm/dxp102",
"article-title": "IL-5-and eosinophil-mediated inflammation: from discovery to therapy",
"author": "Kouro",
"doi-asserted-by": "publisher",
"first-page": "1303",
"journal-title": "Int Immunol.",
"key": "B39",
"volume": "21",
"year": "2009"
},
{
"DOI": "10.1067/mai.2000.109172",
"article-title": "IL-9 expression by human eosinophils: regulation by IL-1β and TNF-α",
"author": "Gounni",
"doi-asserted-by": "publisher",
"first-page": "460",
"journal-title": "J Allergy Clin Immunol.",
"key": "B40",
"volume": "106",
"year": "2000"
},
{
"DOI": "10.1159/000445215",
"article-title": "A player and coordinator: the versatile roles of eosinophils in the immune system",
"author": "Long",
"doi-asserted-by": "publisher",
"first-page": "96",
"journal-title": "Transfus Med Hemotherapy.",
"key": "B41",
"volume": "43",
"year": "2016"
},
{
"DOI": "10.4081/mrm.2021.758",
"article-title": "Differential alterations in peripheral lymphocyte subsets in COVID-19 patients: upregulation of double-positive and double-negative T cells",
"author": "Zahran",
"doi-asserted-by": "publisher",
"first-page": "758",
"journal-title": "Multidiscip Respir Med.",
"key": "B42",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2022.816005",
"article-title": "CD3+ CD4-CD8-(Double-Negative) T Cells in Inflammation, Immune Disorders and Cancer",
"author": "Wu",
"doi-asserted-by": "publisher",
"first-page": "816005",
"journal-title": "Front Immunol.",
"key": "B43",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1038/mi.2015.91",
"article-title": "Double-negative T resident memory cells of the lung react to influenza virus infection via CD11chi dendritic cells",
"author": "Neyt",
"doi-asserted-by": "publisher",
"first-page": "999",
"journal-title": "Mucosal Immunol.",
"key": "B44",
"volume": "9",
"year": "2016"
},
{
"DOI": "10.1124/pharmrev.120.000063",
"article-title": "Pharmacological modulation of immune responses by nutritional components",
"author": "van Daal",
"doi-asserted-by": "publisher",
"first-page": "1369",
"journal-title": "Pharmacol Rev.",
"key": "B45",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.3109/08923973.2010.522195",
"article-title": "Catechin inhibits adhesion and migration of peripheral blood B cells by blocking CD11b",
"author": "Kawai",
"doi-asserted-by": "publisher",
"first-page": "391",
"journal-title": "Immunopharmacol Immunotoxicol.",
"key": "B46",
"volume": "33",
"year": "2011"
}
],
"reference-count": 46,
"references-count": 46,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2022.991873/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Randomized double-blind clinical study in patients with COVID-19 to evaluate the safety and efficacy of a phytomedicine (P2Et)",
"type": "journal-article",
"update-policy": "https://doi.org/10.3389/crossmark-policy",
"volume": "9"
}