Efficacy of remdesivir in hospitalized nonsevere COVID-19 patients in Japan: A large observational study using the COVID-19 Registry Japan
et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2022.02.039, Mar 2021 (preprint)
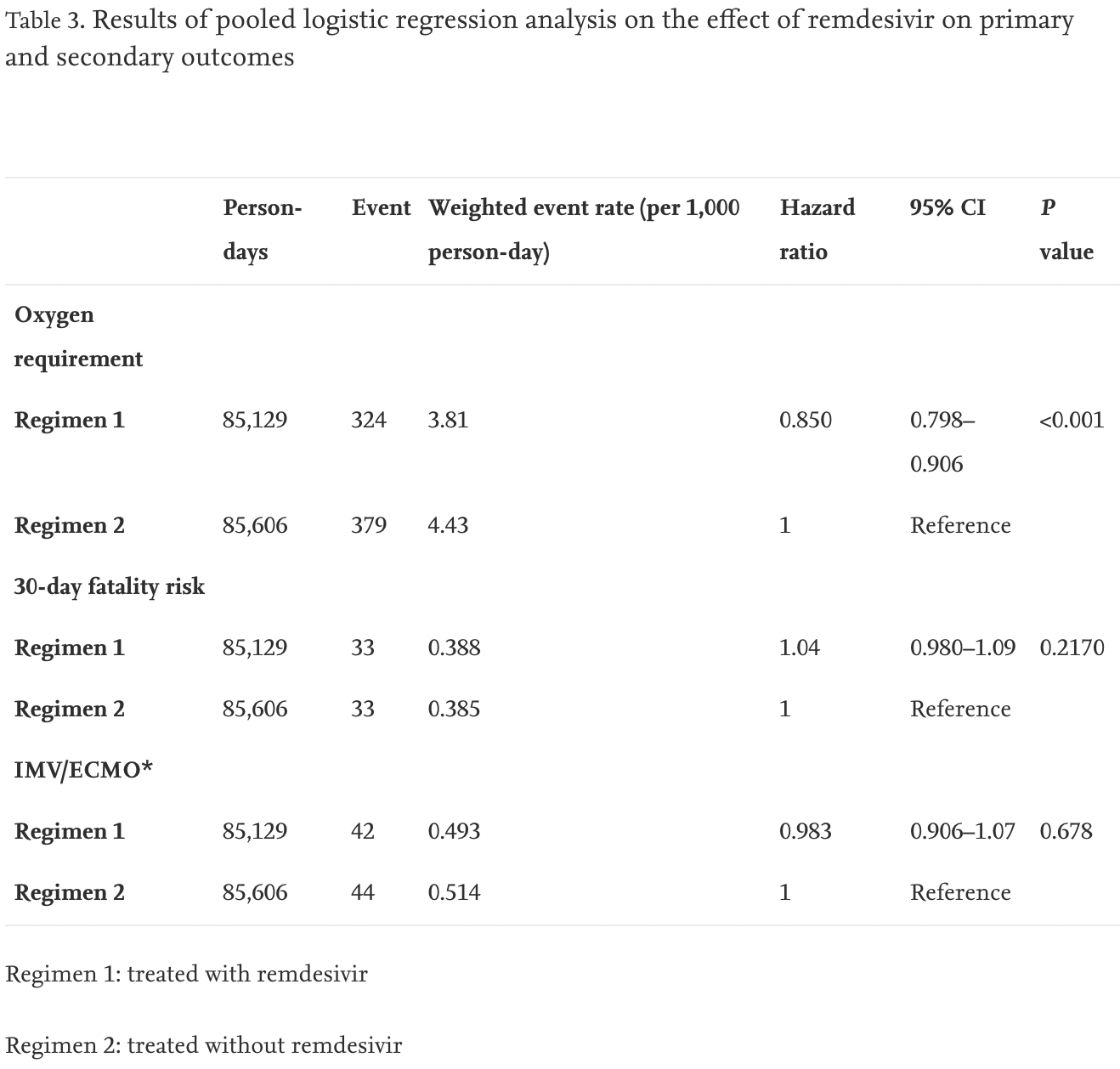
Retrospective database analysis of 12,487 hospitalized patients in Japan, showing lower risk of oxygen requirement, but no significant difference in mortality or ventilation/ECMO.
Gérard, Zhou, Wu, Kamo, Choi, Kim show increased risk of acute kidney injury, Leo, Briciu, Muntean, Petrov show increased risk of liver injury, and Negru, Cheng, Mohammed, Kwok show increased risk of cardiac disorders with remdesivir.
Standard of Care (SOC) for COVID-19 in the study country,
Japan, is very poor with very low average efficacy for approved treatments15.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 4.0% higher, HR 1.04, p = 0.21, treatment 69 of 824 (8.4%), control 285 of 11,663 (2.4%), adjusted per study, day 30.
|
|
risk of mechanical ventilation or ECMO, 1.7% lower, HR 0.98, p = 0.68, treatment 48 of 824 (5.8%), control 98 of 11,663 (0.8%), adjusted per study.
|
|
risk of progression, 15.0% lower, HR 0.85, p = 0.68, treatment 559 of 824 (67.8%), control 1,784 of 11,663 (15.3%), adjusted per study.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Gérard et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145.
2.
Zhou et al., Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679.
3.
Wu et al., Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS, Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828.
4.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
5.
Choi et al., Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0.
6.
Kim et al., Investigating the Safety Profile of Fast‐Track COVID‐19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study, Pharmacoepidemiology and Drug Safety, doi:10.1002/pds.70043.
7.
Leo et al., Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points, Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014.
8.
Briciu et al., Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting, Pathogens, doi:10.3390/pathogens12121453.
9.
Muntean et al., Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre, Pharmaceuticals, doi:10.3390/ph17010003.
10.
Petrov et al., The Effect of Potentially Hepatotoxic Medicinal Products on Alanine Transaminase Levels in COVID-19 Patients: A Case–Control Study, Safety and Risk of Pharmacotherapy, doi:10.30895/2312-7821-2025-458.
11.
Negru et al., Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data, Biomedicines, doi:10.3390/biomedicines13061387.
12.
Cheng et al., Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase, Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z.
13.
Mohammed et al., Bradycardia associated with remdesivir treatment in coronavirus disease 2019 patients: A propensity score-matched analysis, Medicine, doi:10.1097/MD.0000000000044501.
Tsuzuki et al., 10 Mar 2021, retrospective, Japan, peer-reviewed, 21 authors, average treatment delay 6.0 days.
Effectiveness of remdesivir in hospitalized nonsevere patients with COVID-19 in Japan: A large observational study using the COVID-19 Registry Japan
International Journal of Infectious Diseases, doi:10.1016/j.ijid.2022.02.039
This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics This study was approved by the NCGM ethics review (NCGM-G-003494-0). Information regarding opting out of our study is available on the registry website.
Declaration of interests The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
References
Cevik, Kuppalli, Kindrachuk, Peiris, Virology, transmission, and pathogenesis of SARS-CoV-2, BMJ, doi:10.1136/bmj.m3862
Commissioner, The, Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment
Core, R: A language and environment for statistical computing
Cunningham, Vaduganathan, Claggett, Jering, Bhatt et al., Clinical outcomes in young US adults hospitalized with COVID-19, JAMA Intern Med, doi:10.1001/jamainternmed.2020.5313
Hernán, How to estimate the effect of treatment duration on survival outcomes using observational data, BMJ, doi:10.1136/bmj.k182
Hernán, Sauer, Hernández-Díaz, Platt, Shrier, Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses, doi:10.1016/j.jclinepi.2016.04.014
Kitajima, Beigel, Tomashek, Dodd, Mehta et al., Remdesivir for the treatment of COVID-19 -final report, doi:10.1056/NEJMoa2007764.CDC.CoronavirusDisease2019(COVID-
Lighter, Phillips, Hochman, Sterling, Johnson et al., Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission, Clin Infect Dis, doi:10.1093/cid/ciaa415
Matsunaga, Hayakawa, Terada, Ohtsu, Asai et al., Clinical epidemiology of hospitalized patients with COVID-19 in Japan: report of the COVID-19 REGISTRY JAPAN, Clin Infect Dis, doi:10.1093/cid/ciaa1470.MinistryofHealth
Pan, Peto, Henao-Restrepo, Preziosi, Sathiyamoorthy, Repurposed antiviral drugs for COVID-19 -Interim WHO solidarity trial results, N Engl J Med, doi:10.1056/NEJMoa2023184
Petrilli, Jones, Yang, Rajagopalan, Donnell et al., Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study, BMJ, doi:10.1136/bmj.m1966.PharmaceuticalsandMedicalDevicesAgency.Remdesivir
Robins Finkelstein, Correcting for noncompliance and dependent censoring in an AIDS clinical trial with inverse probability of censoring weighted (IPCW) log-rank tests, Biometrics, doi:10.1111/j.0006-341X.2000.00779.x
Rosenbaum, Db, The central role of the propensity score in observational studies for causal effects, Biometrika
Saito, Hayakawa, Mikami, Izumi, Funazaki et al., Investigator initiated clinical trial of remdesivir for the treatment of COVID-19 in Japan, GHM, doi:10.35772/ghm.2020.01106
Spinner, Gottlieb, Criner, López, Cattelan et al., Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2020.16349
Suissa, Dell'aniello, Time-related biases in pharmacoepidemiology, Pharmacoepidemiol Drug Saf, doi:10.1002/pds.5083
Tartof, Qian, Hong, Wei, Nadjafi et al., Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization, Ann Intern Med, doi:10.7326/M20-3742
Williamson, Walker, Bhaskaran, Bacon, Bates et al., Factors associated with COVID-19-related death using OpenSAFELY, Nature, doi:10.1038/s41586-020-2521-4
Yeming, Zhang, Du, Du, Zhao et al., Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial, Lancet, doi:10.1016/S0140-6736(20)31022-9
DOI record:
{
"DOI": "10.1016/j.ijid.2022.02.039",
"ISSN": [
"1201-9712"
],
"URL": "http://dx.doi.org/10.1016/j.ijid.2022.02.039",
"alternative-id": [
"S1201971222001187"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-8504-1244",
"affiliation": [],
"authenticated-orcid": false,
"family": "Tsuzuki",
"given": "Shinya",
"sequence": "first"
},
{
"affiliation": [],
"family": "Hayakawa",
"given": "Kayoko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Uemura",
"given": "Yukari",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3395-9691",
"affiliation": [],
"authenticated-orcid": false,
"family": "Shinozaki",
"given": "Tomohiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Matsunaga",
"given": "Nobuaki",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5171-1828",
"affiliation": [],
"authenticated-orcid": false,
"family": "Terada",
"given": "Mari",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Suzuki",
"given": "Setsuko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Asai",
"given": "Yusuke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kitajima",
"given": "Koji",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saito",
"given": "Sho",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9433-4287",
"affiliation": [],
"authenticated-orcid": false,
"family": "Yamada",
"given": "Gen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shibata",
"given": "Taro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kondo",
"given": "Masashi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Izumi",
"given": "Kazuo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9595-9657",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hojo",
"given": "Masayuki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mizoue",
"given": "Tetsuya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yokota",
"given": "Kazuhisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nakamura-Uchiyama",
"given": "Fukumi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saito",
"given": "Fumitake",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sugiura",
"given": "Wataru",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ohmagari",
"given": "Norio",
"sequence": "additional"
}
],
"container-title": [
"International Journal of Infectious Diseases"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
2,
19
]
],
"date-time": "2022-02-19T15:32:05Z",
"timestamp": 1645284725000
},
"deposited": {
"date-parts": [
[
2022,
2,
19
]
],
"date-time": "2022-02-19T15:32:06Z",
"timestamp": 1645284726000
},
"indexed": {
"date-parts": [
[
2022,
2,
19
]
],
"date-time": "2022-02-19T16:10:45Z",
"timestamp": 1645287045044
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "1201-9712"
}
],
"issued": {
"date-parts": [
[
2022,
2
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
1
]
],
"date-time": "2022-02-01T00:00:00Z",
"timestamp": 1643673600000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 16,
"start": {
"date-parts": [
[
2022,
2,
17
]
],
"date-time": "2022-02-17T00:00:00Z",
"timestamp": 1645056000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971222001187?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971222001187?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
2
]
]
},
"published-print": {
"date-parts": [
[
2022,
2
]
]
},
"publisher": "Elsevier BV",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [
"International Journal of Infectious Diseases"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)",
"General Medicine"
],
"subtitle": [],
"title": [
"Efficacy of remdesivir in hospitalized nonsevere COVID-19 patients in Japan: A large observational study using the COVID-19 Registry Japan"
],
"type": "journal-article"
}