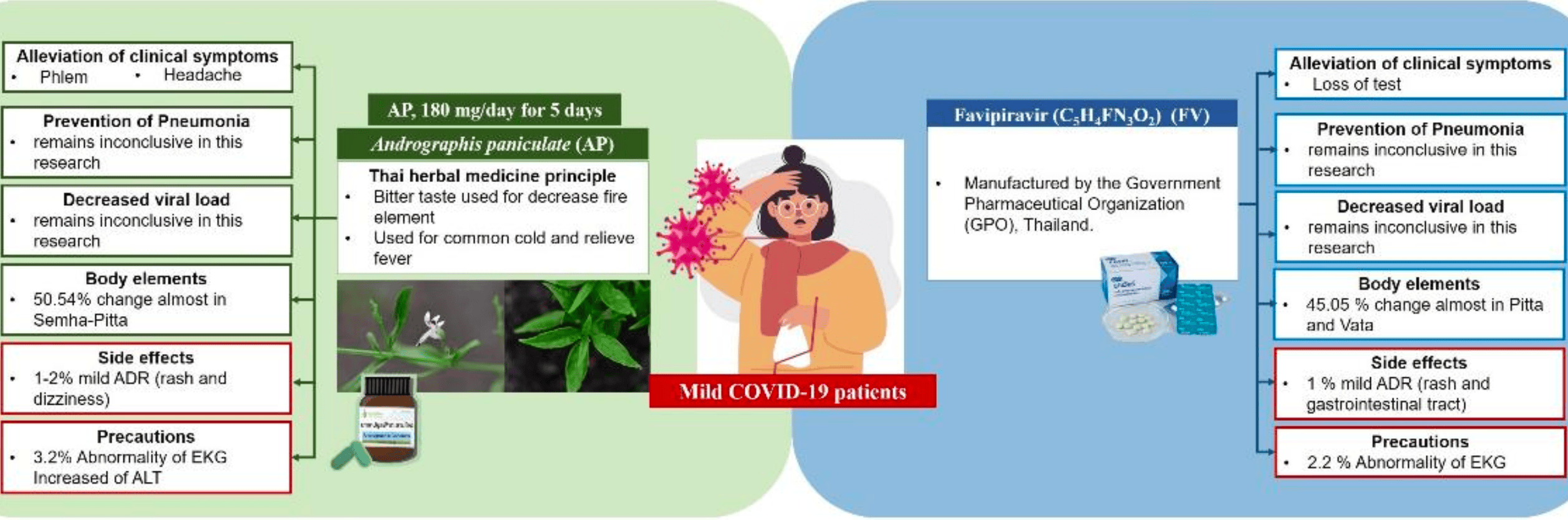
Andrographis paniculata or Favipiravir in Mild COVID-19: A Randomized Control Trial
et al., Phytomedicine Plus, doi:10.1016/j.phyplu.2025.100858, TCTR20240802007, Jul 2025
RCT 184 mild COVID-19 patients in Thailand showing no difference in pneumonia incidence or viral clearance between Andrographis paniculata and favipiravir.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
Study covers andrographolide and favipiravir.
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Sirijatuphat et al., 31 Jul 2025, Double Blind Randomized Controlled Trial, Thailand, peer-reviewed, 13 authors, study period July 2022 - December 2022, trial TCTR20240802007.
Contact: chairatpermpikul@gmail.com.
Andrographis paniculata or Favipiravir in Mild COVID-19: A Randomized Control Trial
Phytomedicine Plus, doi:10.1016/j.phyplu.2025.100858
The study compared the efficacy and safety of Andrographis paniculata capsules versus favipiravir in mild COVID-19 patients. • No patients developed pneumonia; viral clearance and viral load were comparable between groups. • Both treatments achieved similar symptom control and viral reduction with minimal adverse effects. • Andrographis paniculata demonstrated superior improvement in phlegm and headache. • Findings support the potential use of Andrographis paniculata as an alternative treatment for mild COVID-19.
Conflict of interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supporting information
Informed consent Informed consent was obtained from all subjects involved in the study.
Conflict of interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of interests ☒ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. ☐ The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
References
Agrawal, Raju, Udwadia, Guidelines on clinical practice, diagnosis, treatment, and prevention of healthcare-associated infection for COVID-19, Medical Journal Armed Forces India, doi:10.1016/j.mjafi.2020.08.004Coronavirus(COVID-19
Enmozhi, Raja, Sebastine, Joseph, Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: An in silico approach, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2020.1772112
Herbal, None
Hung, Ghula, Aziz, Makram, Tawfik et al., The efficacy and adverse effects of favipiravir on patients with COVID-19: A systematic review and meta-analysis of published clinical trials and observational studies, International Journal of Infectious Diseases, doi:10.1016/j.ijid.2022.04.035
Intharuksa, Arunotayanun, Yooin, Sirisa-Ard, A comprehensive review of Andrographis paniculata (Burm. f.) Nees and its constituents as potential lead compounds for COVID-19 drug discovery, Molecules, doi:10.3390/molecules27144665
Jiang, Sheng, Zhang, Ma, Gao et al., Andrographis paniculata (Burm.f.) Nees and its major constituent andrographolide as potential antiviral agents, Journal of Ethnopharmacology, doi:10.1016/j.jep.2021.113954
Kanokkangsadal, Mingmalairak, Mukkasombat, Kuropakornpong, Worawattananutai et al., Andrographis paniculata extract versus placebo in the treatment of COVID-19: A double-blinded randomized control trial, Research in Pharmaceutical Sciences
Kesheh, Shavandi, Haeri Moghaddam, Effect of herbal compounds on coronavirus: A systematic review and meta-analysis, Virology Journal, doi:10.1186/s12985-022-01808-z
Kim, Kim, Ra, Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19, Clinical Microbiology and Infection, doi:10.1016/j.cmi.2020.04.040
Lim, Chan, Tan, Teh, Mohd Abd Razak et al., Andrographis paniculata (Burm. F.) Wall. ex Nees, andrographolide, and andrographolide analogues as SARS-CoV-2 antivirals? A rapid review, Natural Product Communications, doi:10.1177/1934578X211016610
Ltd, Se, Power calculator for binary outcome non-inferiority trial
Mahajaroensiri, Vannabhum, Leethong, Wanaratna, Inchai et al., Inflammatory cytokines and metabolite changes after high dose of Andrographis paniculata extract: A preliminary study in mild COVID-19 case patients, Journal of Basic and Applied Pharmacology
Prasoppokakorn, Sriphoosanaphan, Nalinthassanai, Roongrawee, Hanboonkunupakarn et al., Efficacy and safety of andrographolide and favipiravir versus favipiravir monotherapy in patients with mild COVID-19 infection: A multicenter randomized controlled trial, OBM Integrative and Complementary Medicine
Prempree, Mungaomklang, Tangkiatkumjai, Phodha, Kwankhao et al., SARS-CoV-2 clearance from Andrographis paniculata, Boesenbergia rotunda, and favipiravir among mild COVID-19 cases in Klong Prem Central Prison during mid-2021: A retrospective study, Outbreak Surveillance Investigation Response Journal
Puriwatthanapong, Chaiyodsilp, Bunsoong, Chaiyodsilp, Tipasaharn et al., Randomized controlled trial to compare the efficacy of Andrographis paniculata powder and favipiravir for the treatment of mild COVID-19, Journal of Thai Traditional and Alternative Medicine
Raj, Maurya, Nair, Marfatia, Hadaye et al., Efficacy and safety of AP-Bio® (KalmCold®) in participants with uncomplicated upper respiratory tract viral infection (common cold) -A phase III, double-blind, parallel group, randomized placebo-controlled trial, Complementary Therapies in Medicine, doi:10.1016/j.ctim.2023.102934
Ratiani, Efficacy of Kan Jang® in patients with mild COVID-19: Interim analysis of a randomized, quadruple-blind, placebo-controlled trial, Pharmaceuticals, doi:10.3390/ph15081013
Ruzhentsova, Oseshnyuk, Soluyanova, Dmitrikova, Mustafaev et al., Phase 3 trial of coronavirus (favipiravir) in patients with mild to moderate COVID-19, American Journal of Translational Research
Salvadori, Jourdain, Krittayaphong, Siripongboonsitti, Kongsaengdao et al., Molnupiravir versus favipiravir in at-risk outpatients with COVID-19: A randomized controlled trial in Thailand, International Journal of Infectious Diseases, doi:10.1016/j.ijid.2024.107021
Saxena, Singh, Kumar, Yadav, Negi et al., A randomized double-blind placebo-controlled clinical evaluation of extract of Andrographis paniculata (KalmCold) in patients with uncomplicated upper respiratory tract infection, Phytomedicine, doi:10.1016/j.phymed.2009.12.001
Shang, Shen, Stub, Zhu, Qiao et al., Adverse effects of andrographolide derivative medications compared to the safe use of herbal preparations of Andrographis paniculata: Results of a systematic review and meta-analysis of clinical studies, Frontiers in Pharmacology, doi:10.3389/fphar.2022.00091
Shi, Huang, Chen, Pi, Hsu et al., Andrographolide and its fluorescent derivative inhibit the main proteases of 2019-nCoV and SARS-CoV through covalent linkage, Biochemical and Biophysical Research Communications, doi:10.1016/j.bbrc.2020.09.020
Sirijatuphat, Manosuthi, Niyomnaitham, Owen, Copeland et al., Early treatment of favipiravir in COVID-19 patients without pneumonia: A multicentre, open-labelled, randomized control study, Emerging Microbes & Infections, doi:10.1080/22221751.2022.2117092
Siripongboonsitti, Ungtrakul, Tawinprai, Auewarakul, Chartisathian et al., Efficacy of Andrographis paniculata extract treatment in mild to moderate COVID-19 patients being treated with favipiravir: A double-blind, randomized, placebo-controlled study (APFaVi trial), Phytomedicine, doi:10.1016/j.phymed.2023.155018
Toh, Ong, Lim, Comparison of seroconversion in children and adults with mild COVID-19, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.1313
Udwadia, Singh, Barkate, Patil, Saiprasad et al., Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial, International Journal of Infectious Diseases, doi:10.1016/j.ijid.2020.11.142
Vannabhum, Mahajaroensiri, Pattanapholkornsakul, Tantiwongsekunakorn, Thippayacharoentam et al., Metabolomics of personalized body elements in Thai traditional medicine response to herbal medicine for body elements balancing in healthy volunteers, Journal of Evidence-Based Complementary & Alternative Medicine, doi:10.1177/21565872231180131
Wanaratna, Leethong, Inchai, Chueawiang, Sriraksa et al., Efficacy and safety of Andrographis paniculata extract in participants with mild COVID-19: A randomized controlled trial, Archives of Internal Medicine Research, doi:10.26502/aimr.0125
DOI record:
{
"DOI": "10.1016/j.phyplu.2025.100858",
"ISSN": [
"2667-0313"
],
"URL": "http://dx.doi.org/10.1016/j.phyplu.2025.100858",
"alternative-id": [
"S2667031325001290"
],
"article-number": "100858",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Andrographis paniculata or Favipiravir in Mild COVID-19: A Randomized Control Trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Phytomedicine Plus"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.phyplu.2025.100858"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2025 Published by Elsevier B.V."
}
],
"author": [
{
"affiliation": [],
"family": "Sirijatuphat",
"given": "Rujipas",
"sequence": "first"
},
{
"affiliation": [],
"family": "Horthongkham",
"given": "Navin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chaimayo",
"given": "Chutikarn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wongprompitak",
"given": "Patimaporn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kongsankum",
"given": "Wuttikrai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Charoenkij",
"given": "Phornnapa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Palo",
"given": "Titchaphorn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mahajaronesiri",
"given": "Suthathip",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nathananwanit",
"given": "Nalinthika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Senawong",
"given": "Sansanee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kantakamalakul",
"given": "Wannee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Akarasereenont",
"given": "Pravit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Permpikul",
"given": "Chairat",
"sequence": "additional"
}
],
"container-title": "Phytomedicine Plus",
"container-title-short": "Phytomedicine Plus",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2025,
7,
28
]
],
"date-time": "2025-07-28T19:02:09Z",
"timestamp": 1753729329000
},
"deposited": {
"date-parts": [
[
2025,
7,
28
]
],
"date-time": "2025-07-28T19:02:14Z",
"timestamp": 1753729334000
},
"funder": [
{
"DOI": "10.13039/501100013238",
"award": [
"R016534001"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100013238",
"id-type": "DOI"
}
],
"name": "Faculty of Medicine Siriraj Hospital, Mahidol University"
}
],
"indexed": {
"date-parts": [
[
2025,
7,
28
]
],
"date-time": "2025-07-28T19:40:07Z",
"timestamp": 1753731607540,
"version": "3.41.2"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
7
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
7,
1
]
],
"date-time": "2025-07-01T00:00:00Z",
"timestamp": 1751328000000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
7,
1
]
],
"date-time": "2025-07-01T00:00:00Z",
"timestamp": 1751328000000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 26,
"start": {
"date-parts": [
[
2025,
7,
27
]
],
"date-time": "2025-07-27T00:00:00Z",
"timestamp": 1753574400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2667031325001290?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2667031325001290?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "100858",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2025,
7
]
]
},
"published-print": {
"date-parts": [
[
2025,
7
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/j.mjafi.2020.08.004",
"article-title": "Favipiravir: A new and emerging antiviral option in COVID-19",
"author": "Agrawal",
"doi-asserted-by": "crossref",
"first-page": "370",
"issue": "4",
"journal-title": "Medical Journal Armed Forces India",
"key": "10.1016/j.phyplu.2025.100858_bib0001",
"volume": "76",
"year": "2020"
},
{
"key": "10.1016/j.phyplu.2025.100858_bib0002",
"unstructured": "Coronavirus (COVID-19). (2020). Retrieved December 21, 2023, from https://who.sprinklr.com/"
},
{
"key": "10.1016/j.phyplu.2025.100858_bib0003",
"series-title": "Guidelines on clinical practice, diagnosis, treatment, and prevention of healthcare-associated infection for COVID-19",
"year": "2021"
},
{
"key": "10.1016/j.phyplu.2025.100858_bib0004",
"series-title": "CPG COVID-19 for public health personnel revision on11 July 2022",
"year": "2022"
},
{
"article-title": "Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: An in silico approach",
"author": "Enmozhi",
"first-page": "3092",
"issue": "9",
"journal-title": "Journal of Biomolecular Structure and Dynamics",
"key": "10.1016/j.phyplu.2025.100858_bib0005",
"volume": "39",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2022.04.035",
"article-title": "The efficacy and adverse effects of favipiravir on patients with COVID-19: A systematic review and meta-analysis of published clinical trials and observational studies",
"author": "Hung",
"doi-asserted-by": "crossref",
"first-page": "217",
"journal-title": "International Journal of Infectious Diseases",
"key": "10.1016/j.phyplu.2025.100858_bib0006",
"volume": "120",
"year": "2022"
},
{
"DOI": "10.3390/molecules27144479",
"article-title": "A comprehensive review of Andrographis paniculata (Burm. f.) Nees and its constituents as potential lead compounds for COVID-19 drug discovery",
"author": "Intharuksa",
"doi-asserted-by": "crossref",
"issue": "14",
"journal-title": "Molecules",
"key": "10.1016/j.phyplu.2025.100858_bib0007",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1016/j.jep.2021.113954",
"article-title": "Andrographis paniculata (Burm.f.) Nees and its major constituent andrographolide as potential antiviral agents",
"author": "Jiang",
"doi-asserted-by": "crossref",
"journal-title": "Journal of Ethnopharmacology",
"key": "10.1016/j.phyplu.2025.100858_bib0008",
"volume": "272",
"year": "2021"
},
{
"DOI": "10.4103/1735-5362.389947",
"article-title": "Andrographis paniculata extract versus placebo in the treatment of COVID-19: A double-blinded randomized control trial",
"author": "Kanokkangsadal",
"doi-asserted-by": "crossref",
"first-page": "592",
"journal-title": "Research in Pharmaceutical Sciences",
"key": "10.1016/j.phyplu.2025.100858_bib0009",
"volume": "18",
"year": "2023"
},
{
"DOI": "10.1186/s12985-022-01808-z",
"article-title": "Effect of herbal compounds on coronavirus: A systematic review and meta-analysis",
"author": "Kesheh",
"doi-asserted-by": "crossref",
"first-page": "87",
"journal-title": "Virology Journal",
"key": "10.1016/j.phyplu.2025.100858_bib0010",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.1016/j.cmi.2020.04.040",
"article-title": "Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "948.e1",
"issue": "7",
"journal-title": "Clinical Microbiology and Infection",
"key": "10.1016/j.phyplu.2025.100858_bib0011",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1177/1934578X211016610",
"article-title": "Andrographis paniculata (Burm. F.) Wall. ex Nees, andrographolide, and andrographolide analogues as SARS-CoV-2 antivirals? A rapid review",
"author": "Lim",
"doi-asserted-by": "crossref",
"issue": "5",
"journal-title": "Natural Product Communications",
"key": "10.1016/j.phyplu.2025.100858_bib0012",
"volume": "16",
"year": "2021"
},
{
"key": "10.1016/j.phyplu.2025.100858_bib0013",
"unstructured": "Ltd. SE. (2012). Power calculator for binary outcome non-inferiority trial. Retrieved August 14, 2021, from https://www.sealedenvelope.com/power/binary-noninferior/"
},
{
"article-title": "Inflammatory cytokines and metabolite changes after high dose of Andrographis paniculata extract: A preliminary study in mild COVID-19 case patients",
"author": "Mahajaroensiri",
"first-page": "21",
"issue": "1",
"journal-title": "Journal of Basic and Applied Pharmacology",
"key": "10.1016/j.phyplu.2025.100858_bib0014",
"volume": "1",
"year": "2021"
},
{
"DOI": "10.21926/obm.icm.2401013",
"article-title": "Efficacy and safety of andrographolide and favipiravir versus favipiravir monotherapy in patients with mild COVID-19 infection: A multicenter randomized controlled trial",
"author": "Prasoppokakorn",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "OBM Integrative and Complementary Medicine",
"key": "10.1016/j.phyplu.2025.100858_bib0015",
"volume": "9",
"year": "2024"
},
{
"DOI": "10.59096/osir.v15i4.262277",
"article-title": "SARS-CoV-2 clearance from Andrographis paniculata, Boesenbergia rotunda, and favipiravir among mild COVID-19 cases in Klong Prem Central Prison during mid-2021: A retrospective study",
"author": "Prempree",
"doi-asserted-by": "crossref",
"first-page": "131",
"issue": "4",
"journal-title": "Outbreak Surveillance Investigation Response Journal",
"key": "10.1016/j.phyplu.2025.100858_bib0016",
"volume": "15",
"year": "2022"
},
{
"article-title": "Randomized controlled trial to compare the efficacy of Andrographis paniculata powder and favipiravir for the treatment of mild COVID-19",
"author": "Puriwatthanapong",
"first-page": "526",
"journal-title": "Journal of Thai Traditional and Alternative Medicine",
"key": "10.1016/j.phyplu.2025.100858_bib0017",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1016/j.ctim.2023.102934",
"article-title": "Efficacy and safety of AP-Bio® (KalmCold®) in participants with uncomplicated upper respiratory tract viral infection (common cold) - A phase III, double-blind, parallel group, randomized placebo-controlled trial",
"author": "Raj",
"doi-asserted-by": "crossref",
"journal-title": "Complementary Therapies in Medicine",
"key": "10.1016/j.phyplu.2025.100858_bib0018",
"volume": "73",
"year": "2023"
},
{
"DOI": "10.3390/ph15081013",
"article-title": "Efficacy of Kan Jang® in patients with mild COVID-19: Interim analysis of a randomized, quadruple-blind, placebo-controlled trial",
"author": "Ratiani",
"doi-asserted-by": "crossref",
"first-page": "1013",
"issue": "8",
"journal-title": "Pharmaceuticals",
"key": "10.1016/j.phyplu.2025.100858_bib0019",
"volume": "15",
"year": "2022"
},
{
"article-title": "Phase 3 trial of coronavirus (favipiravir) in patients with mild to moderate COVID-19",
"author": "Ruzhentsova",
"first-page": "12575",
"journal-title": "American Journal of Translational Research",
"key": "10.1016/j.phyplu.2025.100858_bib0020",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2024.107021",
"article-title": "Molnupiravir versus favipiravir in at-risk outpatients with COVID-19: A randomized controlled trial in Thailand",
"author": "Salvadori",
"doi-asserted-by": "crossref",
"journal-title": "International Journal of Infectious Diseases",
"key": "10.1016/j.phyplu.2025.100858_bib0021",
"volume": "143",
"year": "2024"
},
{
"DOI": "10.1016/j.phymed.2009.12.001",
"article-title": "A randomized double-blind placebo-controlled clinical evaluation of extract of Andrographis paniculata (KalmCold) in patients with uncomplicated upper respiratory tract infection",
"author": "Saxena",
"doi-asserted-by": "crossref",
"first-page": "178",
"issue": "3-4",
"journal-title": "Phytomedicine",
"key": "10.1016/j.phyplu.2025.100858_bib0022",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2022.773282",
"article-title": "Adverse effects of andrographolide derivative medications compared to the safe use of herbal preparations of Andrographis paniculata: Results of a systematic review and meta-analysis of clinical studies",
"author": "Shang",
"doi-asserted-by": "crossref",
"first-page": "91",
"journal-title": "Frontiers in Pharmacology",
"key": "10.1016/j.phyplu.2025.100858_bib0023",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/j.bbrc.2020.08.086",
"article-title": "Andrographolide and its fluorescent derivative inhibit the main proteases of 2019-nCoV and SARS-CoV through covalent linkage",
"author": "Shi",
"doi-asserted-by": "crossref",
"first-page": "467",
"issue": "3",
"journal-title": "Biochemical and Biophysical Research Communications",
"key": "10.1016/j.phyplu.2025.100858_bib0024",
"volume": "533",
"year": "2020"
},
{
"DOI": "10.1080/22221751.2022.2117092",
"article-title": "Early treatment of favipiravir in COVID-19 patients without pneumonia: A multicentre, open-labelled, randomized control study",
"author": "Sirijatuphat",
"doi-asserted-by": "crossref",
"first-page": "2197",
"issue": "1",
"journal-title": "Emerging Microbes & Infections",
"key": "10.1016/j.phyplu.2025.100858_bib0025",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1016/j.phymed.2023.155018",
"article-title": "Efficacy of Andrographis paniculata extract treatment in mild to moderate COVID-19 patients being treated with favipiravir: A double-blind, randomized, placebo-controlled study (APFaVi trial)",
"author": "Siripongboonsitti",
"doi-asserted-by": "crossref",
"journal-title": "Phytomedicine",
"key": "10.1016/j.phyplu.2025.100858_bib0026",
"volume": "119",
"year": "2023"
},
{
"DOI": "10.1001/jamanetworkopen.2022.1313",
"article-title": "Comparison of seroconversion in children and adults with mild COVID-19",
"author": "Toh",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "JAMA Network Open",
"key": "10.1016/j.phyplu.2025.100858_bib0028",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1016/j.ijid.2020.11.142",
"article-title": "Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial",
"author": "Udwadia",
"doi-asserted-by": "crossref",
"first-page": "62",
"journal-title": "International Journal of Infectious Diseases",
"key": "10.1016/j.phyplu.2025.100858_bib0029",
"volume": "103",
"year": "2021"
},
{
"article-title": "Metabolomics of personalized body elements in Thai traditional medicine response to herbal medicine for body elements balancing in healthy volunteers",
"author": "Vannabhum",
"journal-title": "Journal of Evidence-Based Complementary & Alternative Medicine",
"key": "10.1016/j.phyplu.2025.100858_bib0030",
"volume": "2023",
"year": "2023"
},
{
"DOI": "10.26502/aimr.0125",
"article-title": "Efficacy and safety of Andrographis paniculata extract in participants with mild COVID-19: A randomized controlled trial",
"author": "Wanaratna",
"doi-asserted-by": "crossref",
"first-page": "423",
"issue": "3",
"journal-title": "Archives of Internal Medicine Research",
"key": "10.1016/j.phyplu.2025.100858_bib0031",
"volume": "5",
"year": "2022"
},
{
"key": "10.1016/j.phyplu.2025.100858_bib0032",
"series-title": "WHO coronavirus (COVID-19) dashboard",
"year": "2023"
},
{
"key": "10.1016/j.phyplu.2025.100858_bib0033",
"series-title": "WHO Thailand weekly situation update no. 240 2022",
"year": "2022"
},
{
"key": "10.1016/j.phyplu.2025.100858_bib0034",
"series-title": "WHO Thailand weekly situation update no. 243 2022",
"year": "2022"
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2667031325001290"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Andrographis paniculata or Favipiravir in Mild COVID-19: A Randomized Control Trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy"
}
sirijatuphat4