Phase-II RCT Convalescent Plasma Transfusion in Severe COVID-19 Patients -Evaluation of Efficacy and Tolerability
et al., Saudi Journal of Medicine, doi:10.36348/sjm.2025.v10i03.00X, Mar 2025
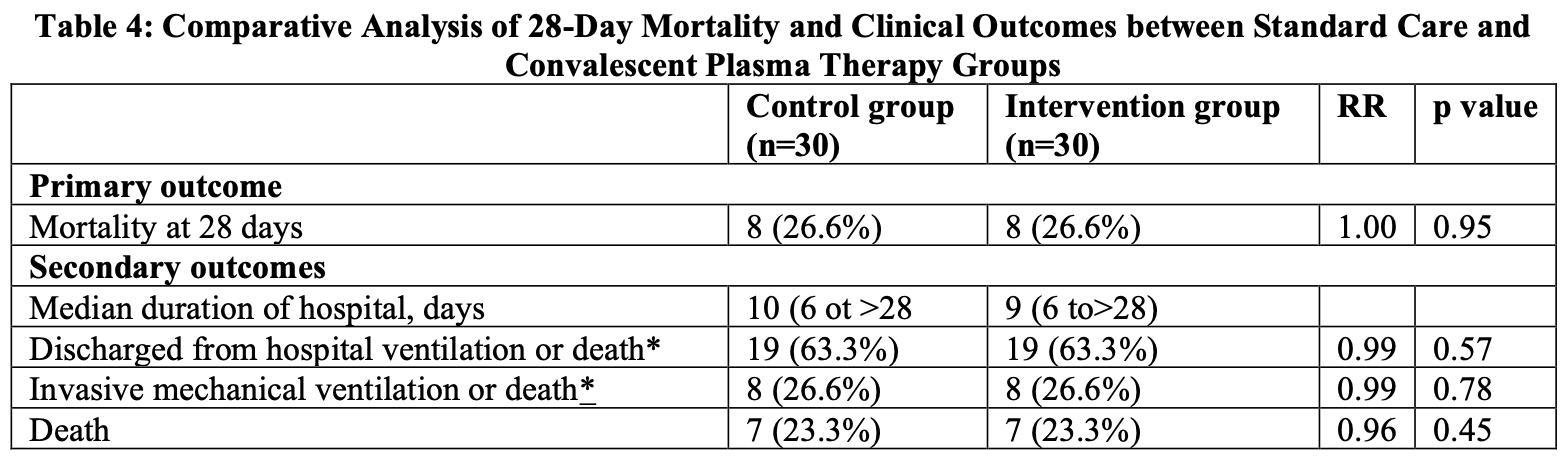
RCT 60 severe COVID-19 patients showing no benefit with convalescent plasma.
|
risk of death, no change, RR 1.00, p = 1.00, treatment 8 of 30 (26.7%), control 8 of 30 (26.7%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Shaheen et al., 31 Mar 2025, Randomized Controlled Trial, Bangladesh, peer-reviewed, mean age 51.7, 9 authors, study period June 2020 - July 2021.
Contact: shaheen@bsmmu.edu.bd.
Phase-II RCT Convalescent Plasma Transfusion in Severe COVID-19 Patients -Evaluation of Efficacy and Tolerability
doi:10.36348/sjm.2025.v10i03.00X
Background: The COVID-19 pandemic necessitated exploration of convalescent plasma (CP) therapy. This phase-II RCT evaluated CP's efficacy in 30 severe COVID-19 patients (June 2020-July 2021), comparing standard care (n=30) with CPadded therapy (n=30). Despite historical success in viral outbreaks, evidence remained conflicting. We assessed 28-day mortality, clinical improvement, and safety, addressing gaps in donor antibody variability and timing. Objective: To determine therapeutic Role of Convalescent Plasma (CP) therapy in the treatment of severe COVID-19. Methods and Procedure: It was a Randomized Controlled phase-II Trial which was carried out at COVID-19 unit and ICU Bangabandhu Sheikh Mujib Medical University, Dhaka from 01June 2020 to 31July 2021. Plasma was collected and supplied in the department of Transfusion Medicine of BSMMU and transfused in patient at ICU, BSMMU. After proper evaluation 30 healthy donors required amount of convalescent plasma of COVID-19 was collected by continuous flow cell separator. The collected convalescent plasma was transfused to; 30 (INTERVENTION ARM) severe ill patients receiving standard treatment protocol admitted in ICU, BSMMU, Dhaka. Then the improvement of these patients was observed and another 30(control) patients receiving standard treatment protocol only and comparison was made. Before administration of the plasma it was screened for RCT-PCR for covid-19. HBsAg, Anti-HCV, HIV and other infections. Results: The study included 60 COVID-19 patients (30 control, 30 intervention) with comparable baseline characteristics (mean age 51-53 years; 40% vs. 56.6% males). The intervention group showed significantly higher baseline D-dimer (4.3 vs. 0.5 µg/mL, p<0.001) and ferritin (1045 vs. 631 ng/mL, p=0.049). Both groups had similar 28-day mortality (26.6%, RR=1.00, p=0.95), hospitalization duration (10 vs. 9 days), and discharge rates (63.3%). Clinical parameters improved over time, with mortality declining from 7.1% (Week-I) to 4.5% (Week-IV). Conclusion: In conclusion, this phase-II randomized controlled trial demonstrated that convalescent plasma (CP) therapy did not significantly improve 28-day mortality or other clinical outcomes in severe COVID-19 patients compared to standard care alone.
Conflict of interest: None declared
References
Agarwal, Mukherjee, Kumar, Convalescent plasma in the management of moderate covid-19 in adults in India: open-label phase II multicentre randomised controlled trial 7 (PLACID Trial), BMJ, doi:10.1136/bmj.m3939
Bloch, Deployment of convalescent plasms for the prevention and treatment of COVID-19, J clin Invest
Bloch, Shoham, Casadevall, Deployment of convalescent plasma for the prevention and treatment of COVID-19, J Clin Invest, doi:10.1172/JCI138745
Bégin, Callum, Jamula, Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial (CONCOR-1), JAMA, doi:10.1001/jama.2021.18178
Casadevall, Joyner, Pirofski, SARS-CoV-2 viral load and antibody responses: the case for convalescent plasma therapy, J Clin Invest, doi:10.1172/JCI139760
Casadevall, None, al Antimicrob Agents Chemother
Casadevall, Pirofski, The convalescent sera option for containing COVID-19, J Clin Invest, doi:10.1172/JCI138003
Estcourt, Turgeon, Mcquilten, Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial, Nat Med, doi:10.1038/s41591-021-01488-2
Focosi, Franchini, Pirofski, COVID-19 convalescent plasma and clinical trials: understanding conflicting outcomes, Clin Microbiol Rev, doi:10.1128/CMR.00200-21
Janiaud, Axfors, Schmitt, Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis, JAMA, doi:10.1001/jama.2021.2747
Joyner, Bruno, Klassen, Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients, Mayo Clin Proc, doi:10.1016/j.mayocp.2020.06.028
Joyner, Wright, Fairweather, Early safety indicators of COVID-19 convalescent plasma in 5,000 patients, J Clin Invest, doi:10.1172/JCI140200
Klassen, Senefeld, Johnson, The effect of convalescent plasma therapy on mortality among patients with COVID-19: systematic review and meta-analysis, Mayo Clin Proc, doi:10.1016/j.mayocp.2021.02.008
Li, Zhang, Hu, Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2020.10044
Libster, Marc, Wappner, Early high-titer plasma therapy to prevent severe Covid-19 in older adults, N Engl J Med, doi:10.1056/NEJMoa2033700
Mair-Jenkins, Saavedra-Campos, Baillie, The effectiveness of convalescent plasma and hyperimmune immunoglobulin for treating severe acute respiratory infections of viral etiology: a systematic review and meta-analysis, J Infect Dis, doi:10.1093/infdis/jiu396
Piechotta, Iannizzi, Chai, Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review, Cochrane Database Syst Rev, doi:10.1002/14651858.CD013600.pub4
Rojas, Rodríguez, Monsalve, Convalescent plasma in Covid-19: possible mechanisms of action, Autoimmun Rev, doi:10.1016/j.autrev.2020.102554
Salazar, Christensen, Graviss, Early high-titer convalescent plasma therapy for COVID-19: a randomized controlled trial, J Clin Invest, doi:10.1172/JCI152740
Salazar, Christensen, Graviss, Significantly decreased mortality in a large cohort of coronavirus disease 2019 (COVID-19) patients transfused early with convalescent plasma containing high-titer anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein IgG, Am J Pathol, doi:10.1016/j.ajpath.2020.10.008
Salazar, Perez, Ashraf, Treatment of coronavirus disease 2019 (COVID-19) patients with convalescent plasma, Am J Pathol, doi:10.1016/j.ajpath.2020.05.014
Shen, Wang, Zhao, Treatment of 5 critically ill patients with COVID-19 with convalescent plasma, JAMA, doi:10.1001/jama.2020.4783
Simonovich, Pratx, Scibona, A randomized trial of convalescent plasma in Covid-19 severe pneumonia, N Engl J Med, doi:10.1056/NEJMoa2031304
Tiberghien, De Lamballerie, Morel, Collecting and evaluating convalescent plasma for COVID-19 treatment: why and how?, Vox Sang, doi:10.1111/vox.12926