Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial
et al., Nature Medicine, doi:10.1038/s41591-021-01488-2, CONCOR-1, NCT04348656, Sep 2021
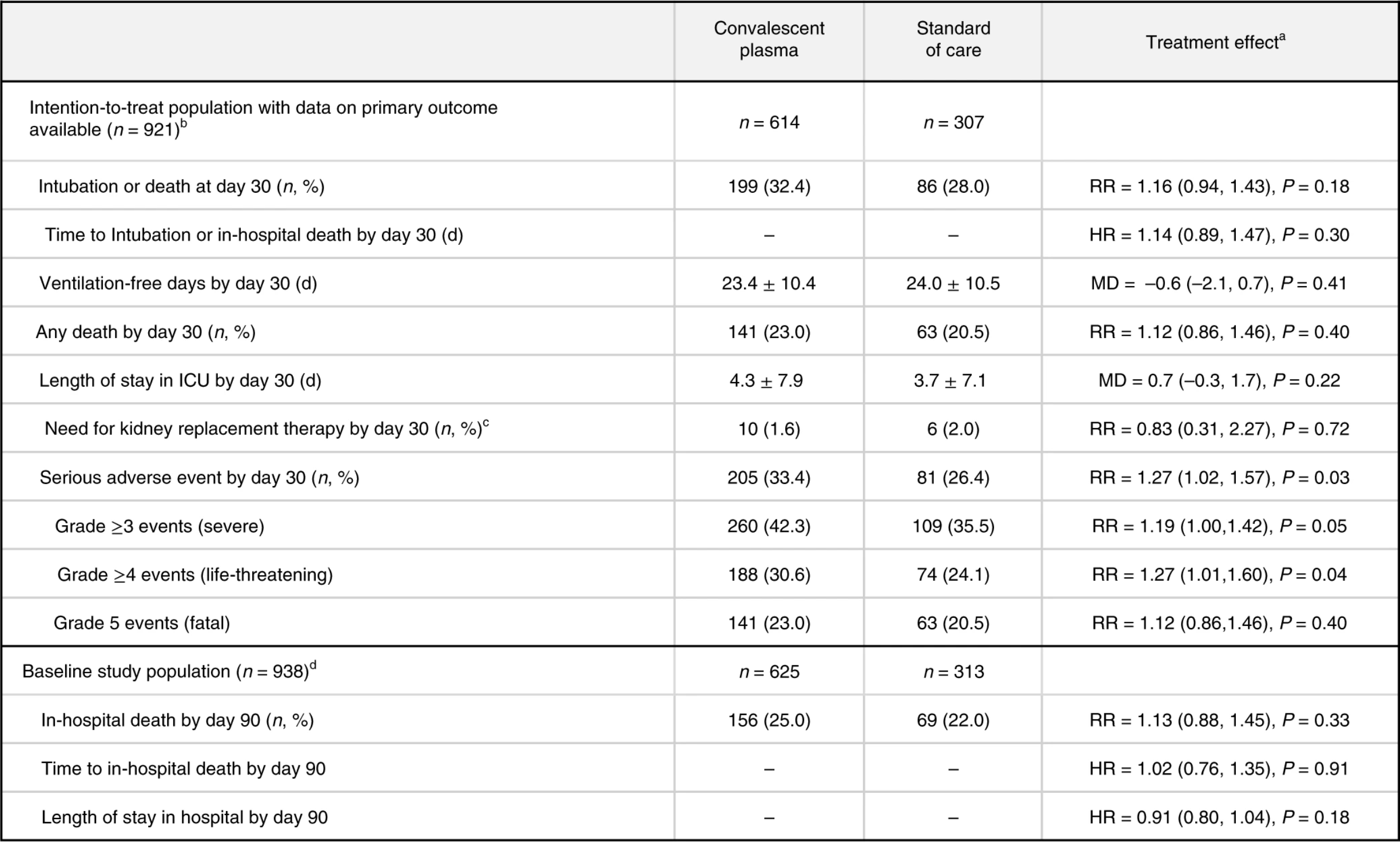
RCT 940 hospitalized patients, 614 assigned to convalescent plasma, showing no significant differences.
|
risk of death, 13.0% higher, RR 1.13, p = 0.33, treatment 156 of 625 (25.0%), control 69 of 313 (22.0%), day 90.
|
|
risk of death, 12.0% higher, RR 1.12, p = 0.40, treatment 141 of 614 (23.0%), control 63 of 307 (20.5%), day 30.
|
|
risk of death/intubation, 16.0% higher, RR 1.16, p = 0.18, treatment 199 of 614 (32.4%), control 86 of 307 (28.0%), primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Bégin et al., 9 Sep 2021, Randomized Controlled Trial, multiple countries, peer-reviewed, 33 authors, study period 14 May, 2020 - 29 January, 2021, average treatment delay 8.0 days, trial NCT04348656 (history) (CONCOR-1).
Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial
Nature Medicine, doi:10.1038/s41591-021-01488-2
T he immune response after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection results in the formation of antibodies that can interfere with viral replication and infection of host cells in over 95% of patients 1 . Based on previous experience in other viral infections 2 , the use of convalescent plasma has been proposed as a therapeutic form of passive immunization for patients with acute 4 ). Early in the pandemic, several small randomized trials found no difference in clinical outcomes 5-8 . In the United States, an Extended Access Program outside of a controlled trial led to the use of convalescent plasma in over half a million patients. Data from these patients showed that the transfusion of plasma with high anti-SARS-CoV-2 antibody levels was associated with a lower risk of death in non-intubated patients compared to lower antibody levels; however, this study lacked a control group 9 . The RECOVERY trial was a large randomized trial in 11,558 hospitalized patients that found that the risk of death after the administration of high-titer plasma was not different from standard of care 10 . The Convalescent Plasma for COVID-19 Respiratory Illness (CONCOR-1) trial was a multi-center, international, open-label, randomized controlled trial designed to assess the effectiveness and safety of COVID-19 convalescent plasma in hospitalized patients. The trial used plasma collected from four blood suppliers with a range of anti-SARS-CoV-2 antibody levels. The variability in antibody titers allowed for a characterization of the effect-modifying role of functional and quantitative antibodies on the primary outcome (intubation or death at 30 d).
Results Patients. This trial was stopped at the planned interim analysis because the conditional power estimate was 1.6% (below the stopping criterion of 20%). Between 14 May 2020 and 29 January 2021, 940 patients were randomized (2:1) to convalescent plasma or standard of care in 72 hospital sites in Canada, the United States and Brazil (Fig. 1 and Supplementary Table 1 ). Two patients randomized
Online content Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at https://doi.org/10.1038/ s41591-021-01488-2. Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Methods Trial design and oversight. CONCOR-1 was an investigator-initiated, multi-center, open-label, randomized controlled trial conducted at 72 hospital sites in Canada, the United States and Brazil 34 . Eligible patients were randomly assigned to receive either convalescent plasma or standard of care. The ). An independent data safety monitoring committee performed trial oversight and made recommendations after review of safety reports planned at every 100 patients and at the planned interim analysis based on the first 600 patients. External monitoring was performed at all sites to assess protocol adherence, reporting of adverse events and accuracy of data entry. Full details of the study design, conduct, oversight and analyses are provided in the protocol and statistical analysis plan, which are available online. Participants. Eligible participants were (1) ≥16 years of age in Canada or ≥18 years of age in the United States and Brazil; (2) admitted to the hospital ward with confirmed..
References
Abe, A simple protein-based surrogate neutralization assay for SARS-CoV-2, JCI Insight
Agarwal, Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial), Brit. Med. J
Alqahtani, Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease, Sci. Rep
Anand, High-throughput detection of antibodies targeting the SARS-CoV-2 spike in longitudinal convalescent plasma samples, Transfusion
Anand, Longitudinal analysis of humoral immunity against SARS-CoV-2 spike in convalescent individuals up to eight months post-symptom onset, Cell Rep. Med
Avendaño-Solà, Convalescent plasma for COVID-19: a multicenter, randomized clinical trial, doi:10.1101/2020.08.26.20182444v3
Bajpai, efficacy of convalescent plasma therapy compared to fresh frozen plasma in severely ill COVID-19 patients: a pilot randomized controlled trial, doi:10.1101/2020.10.25.20219337v1
Beaudoin-Bussieres, Decline of humoral responses against SARS-CoV-2 spike in convalescent individuals, doi:10.1101/2020.07.09.194639v1
Bennett-Guerrero, Severe acute respiratory syndrome coronavirus 2 convalescent plasma versus standard plasma in Coronavirus Disease 2019 infected hospitalized patients in New York: a double-blind randomized trial, Crit. Care Med
Blackall, Rapid establishment of a COVID-19 convalescent plasma program in a regional health care delivery network, Transfusion
Brunet-Ratnasingham, Integrated immunovirological profiling validates plasma SARS-CoV-2 RNA as an early predictor of COVID-19 mortality, doi:10.1101/2021.03.18.21253907v1
Budhai, How did we rapidly implement a convalescent plasma program?, Transfusion
Casadevall, Joyner, Pirofski, Neutralizing antibody LY-CoV555 for outpatient Covid-19, N. Engl. J. Med
Devasenapathy, Efficacy and safety of convalescent plasma for severe COVID-19 based on evidence in other severe respiratory viral infections: a systematic review and meta-analysis, CMAJ
Development, Team, R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing
Estcourt, Convalescent plasma in critically ill patients with Covid-19, doi:10.1101/2021.06.11.21258760v1
General, None
Gharbharan, Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection, Nat. Commun
Goss, Gulick, Vaamonde, Oriela Cuevas 6 , Wanda Lafresne 6
Hamdy Salman, Ail Mohamed, Efficacy and safety of transfusing plasma from COVID-19 survivors to COVID-19 victims with severe illness. A double-blinded controlled preliminary study, Egypt. J. Anaesth
Hamilton, Lee, Arnold, Lilford, Hemming, Is convalescent plasma futile in COVID-19? A Bayesian re-analysis of the RECOVERY randomized controlled trial, Int. J. Infect. Dis
Harris, Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support, J. Biomed. Inform
Haynes, Immune-correlates analysis of an HIV-1 vaccine efficacy trial, N. Engl. J. Med
Joyner, Convalescent plasma antibody levels and the risk of death from Covid-19, N. Engl. J. Med
Körper, High dose convalescent plasma in COVID-19: results from the randomized trial CAPSID, doi:10.1101/2021.05.10.21256192v1
Li, Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial, JAMA
Libster, Early high-titer plasma therapy to prevent severe Covid-19 in older adults, N. Engl. J. Med
Matukas, Charles, Francoeur, Lauzier, Annie La Haye 1 , Vincent Lague 1
Mendoza, Manguiat, Wood, Drebot, Two detailed plaque assay protocols for the quantification of infectious SARS-CoV-2, Curr. Protoc. Microbiol
Narick, Triulzi, Yazer, Transfusion-associated circulatory overload after plasma transfusion, Transfusion
Nature, None
O'brien, Fleming, A multiple testing procedure for clinical trials, Biometrics
O'donnell, A randomized double-blind controlled trial of convalescent plasma in adults with severe COVID-19, J. Clin. Invest
Perreault, Waning of SARS-CoV-2 RBD antibodies in longitudinal convalescent plasma samples within 4 months after symptom onset, Blood
Piechotta, Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review, Cochrane Database Syst. Rev
Ray, Clinical and immunological benefits of convalescent plasma therapy in severe COVID-19: insights from a single center open label randomised control trial, doi:10.1101/2020.11.25.20237883v1
Robinson, Liu, Wong, Sutharsan Suntharalingam 50
Schafer, Antibody potency, effector function, and combinations in protection and therapy for SARS-CoV-2 infection in vivo, J. Exp. Med
Seow, Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans, Nat. Microbiol
Simonovich, A randomized trial of convalescent plasma in Covid-19 severe pneumonia, N. Engl. J. Med
Suryadevara, Neutralizing and protective human monoclonal antibodies recognizing the N-terminal domain of the SARS-CoV-2 spike protein, Cell
Taborda, Rivera, Zaragoza, Casadevall, More is not necessarily better: prozone-like effects in passive immunization with IgG, J. Immunol
Tauzin, A single BNT162b2 mRNA dose elicits antibodies with Fc-mediated effector functions and boost pre-existing humoral and T cell responses, doi:10.1101/2021.03.18.435972v1
The, Group, Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial, Lancet
Tomaras, Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG, Proc. Natl Acad. Sci
Ullah, Live imaging of SARS-CoV-2 infection in mice reveals neutralizing antibodies require Fc function for optimal efficacy, doi:10.1101/2021.03.22.436337v1.full
Winkler, Human neutralizing antibodies against SARS-CoV-2 require intact Fc effector functions for optimal therapeutic protection, Cell
Wood, Estcourt, Mcquilten, How should we use convalescent plasma therapies for the management of COVID-19?, Blood
Wood, Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models, J. R. Stat. Soc. Ser. B (Methodol.)
Wood, Generalized Additive Models: An Introduction with R, Second Edition
Zohar, Compromised humoral functional evolution tracks with SARS-CoV-2 mortality, Cell
DOI record:
{
"DOI": "10.1038/s41591-021-01488-2",
"ISSN": [
"1078-8956",
"1546-170X"
],
"URL": "http://dx.doi.org/10.1038/s41591-021-01488-2",
"abstract": "<jats:title>Abstract</jats:title><jats:p>The efficacy of convalescent plasma for coronavirus disease 2019 (COVID-19) is unclear. Although most randomized controlled trials have shown negative results, uncontrolled studies have suggested that the antibody content could influence patient outcomes. We conducted an open-label, randomized controlled trial of convalescent plasma for adults with COVID-19 receiving oxygen within 12 d of respiratory symptom onset (<jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://clinicaltrials.gov/ct2/show/NCT04348656\">NCT04348656</jats:ext-link>). Patients were allocated 2:1 to 500 ml of convalescent plasma or standard of care. The composite primary outcome was intubation or death by 30 d. Exploratory analyses of the effect of convalescent plasma antibodies on the primary outcome was assessed by logistic regression. The trial was terminated at 78% of planned enrollment after meeting stopping criteria for futility. In total, 940 patients were randomized, and 921 patients were included in the intention-to-treat analysis. Intubation or death occurred in 199/614 (32.4%) patients in the convalescent plasma arm and 86/307 (28.0%) patients in the standard of care arm—relative risk (RR) = 1.16 (95% confidence interval (CI) 0.94–1.43,<jats:italic>P</jats:italic> = 0.18). Patients in the convalescent plasma arm had more serious adverse events (33.4% versus 26.4%; RR = 1.27, 95% CI 1.02–1.57,<jats:italic>P</jats:italic> = 0.034). The antibody content significantly modulated the therapeutic effect of convalescent plasma. In multivariate analysis, each standardized log increase in neutralization or antibody-dependent cellular cytotoxicity independently reduced the potential harmful effect of plasma (odds ratio (OR) = 0.74, 95% CI 0.57–0.95 and OR = 0.66, 95% CI 0.50–0.87, respectively), whereas IgG against the full transmembrane spike protein increased it (OR = 1.53, 95% CI 1.14–2.05). Convalescent plasma did not reduce the risk of intubation or death at 30 d in hospitalized patients with COVID-19. Transfusion of convalescent plasma with unfavorable antibody profiles could be associated with worse clinical outcomes compared to standard care.</jats:p>",
"alternative-id": [
"1488"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "18 June 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2 August 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "9 September 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Change Date",
"name": "change_date",
"order": 4,
"value": "12 January 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Change Type",
"name": "change_type",
"order": 5,
"value": "Correction"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Change Details",
"name": "change_details",
"order": 6,
"value": "A Correction to this paper has been published:"
},
{
"URL": "https://doi.org/10.1038/s41591-021-01667-1",
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Change Details",
"name": "change_details",
"order": 7,
"value": "https://doi.org/10.1038/s41591-021-01667-1"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-9089-4604",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bégin",
"given": "Philippe",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-0943-8853",
"affiliation": [],
"authenticated-orcid": false,
"family": "Callum",
"given": "Jeannie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jamula",
"given": "Erin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cook",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Heddle",
"given": "Nancy M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tinmouth",
"given": "Alan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zeller",
"given": "Michelle P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beaudoin-Bussières",
"given": "Guillaume",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Amorim",
"given": "Luiz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bazin",
"given": "Renée",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Loftsgard",
"given": "Kent Cadogan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carl",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chassé",
"given": "Michaël",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cushing",
"given": "Melissa M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daneman",
"given": "Nick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Devine",
"given": "Dana V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dumaresq",
"given": "Jeannot",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fergusson",
"given": "Dean A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gabe",
"given": "Caroline",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2145-148X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Glesby",
"given": "Marshall J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Na",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Yang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McGeer",
"given": "Allison",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Robitaille",
"given": "Nancy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sachais",
"given": "Bruce S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scales",
"given": "Damon C.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4959-225X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Schwartz",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shehata",
"given": "Nadine",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5675-8791",
"affiliation": [],
"authenticated-orcid": false,
"family": "Turgeon",
"given": "Alexis F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wood",
"given": "Heidi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zarychanski",
"given": "Ryan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Finzi",
"given": "Andrés",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marceau",
"given": "Danièle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huang",
"given": "Andy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carr",
"given": "Holly",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lin",
"given": "Yulia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lall",
"given": "Rosemarie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Graham",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arsenault",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sales",
"given": "Valerie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sidhu",
"given": "Davinder",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Semret",
"given": "Makeda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hamm",
"given": "Caroline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arhanchiague",
"given": "Eneko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Solh",
"given": "Ziad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Srour",
"given": "Nadim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Soliman",
"given": "Karim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yee",
"given": "Colin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Laroche",
"given": "Vincent",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nahirniak",
"given": "Susan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Greenaway",
"given": "Christina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pai",
"given": "Menaka",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Côté",
"given": "Andréanne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tsang",
"given": "Jennifer L. Y.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cserti-Gazdewich",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Talbot",
"given": "Danielle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Poulin",
"given": "Sébastien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guimaraes",
"given": "Rodrigo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rushton-Marovac",
"given": "Moira",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Langlois",
"given": "Alexandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ning",
"given": "Shuoyan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shih",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boileau",
"given": "Mélissa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singh",
"given": "Harjot",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ledingham",
"given": "Donna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ponnampalam",
"given": "Arjuna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yan",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Prokopchuk-Gauk",
"given": "Oksana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Poirier",
"given": "André",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Girouard",
"given": "Gabriel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pavenski",
"given": "Katerina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Drouin",
"given": "Olivier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harris",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Durand",
"given": "Madeleine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rimmer",
"given": "Emily",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ovakim",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ménard",
"given": "François",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cuccarolo",
"given": "Glenna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carruthers",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lucier",
"given": "Kayla",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arsenault",
"given": "Valérie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Auclair",
"given": "Marie-Christine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Avram",
"given": "Meda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brassard",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cerro",
"given": "Sabrina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martinez",
"given": "Véronica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morin",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saint-Jacques",
"given": "Marie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Veillette",
"given": "Maxime",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Armali",
"given": "Chantal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kron",
"given": "Amie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Modi",
"given": "Dimpy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duncan",
"given": "Joanne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Justumus",
"given": "Pauline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "John",
"given": "Melanie St",
"sequence": "additional"
},
{
"affiliation": [],
"family": "St-Onge",
"given": "Geneviève",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hadzi-Tosev",
"given": "Milena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dion",
"given": "Pierre-Marc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McGillivary",
"given": "Lawrence",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Moulliac",
"given": "Andre Valleteau",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nyman",
"given": "Sheila A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Perilli",
"given": "Stephanie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Van Vliet",
"given": "Paulette Jean",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lane",
"given": "Shannon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pavenski",
"given": "Katerina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pereira",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sirotich",
"given": "Emily",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abelson",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Greene",
"given": "Saara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khandelwal",
"given": "Aditi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thakar",
"given": "Swarni",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Longo",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Anand",
"given": "Sai Priya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Benlarbi",
"given": "Mehdi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bourassa",
"given": "Catherine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boutin",
"given": "Marianne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Descôteaux-Dinelle",
"given": "Jade",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gendron-Lepage",
"given": "Gabrielle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goyette",
"given": "Guillaume",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Laumaea",
"given": "Annemarie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Medjahed",
"given": "Halima",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Prévost",
"given": "Jérémie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Richard",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kaufmann",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brunet-Ratnasingham",
"given": "Elsa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chaumont",
"given": "Nicolas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Drebot",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Robinson",
"given": "Alyssia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mendoza",
"given": "Emelissa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dimitrova",
"given": "Kristina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Manguiat",
"given": "Kathy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Phillipson",
"given": "Clark",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chan",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Evans",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lin",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boyer",
"given": "Lucie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cloutier",
"given": "Marc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Drouin",
"given": "Mathieu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ducas",
"given": "Éric",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dussault",
"given": "Nathalie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fournier",
"given": "Marie-Josée",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Landy",
"given": "Patricia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nolin",
"given": "Marie-Ève",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Perreault",
"given": "Josée",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tremblay",
"given": "Tony",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nazy",
"given": "Ishac",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xie",
"given": "Feng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wong",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Silverio",
"given": "Gus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Walkus",
"given": "Kristin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barton",
"given": "Mikaela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haveman",
"given": "Katherine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mueller",
"given": "Darlene",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scott",
"given": "Ashley",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moher",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wood",
"given": "Gordon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roarty",
"given": "Tracey",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Auld",
"given": "Fiona",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carney",
"given": "Gayle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thomson",
"given": "Virginia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Onell",
"given": "Rodrigo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Walley",
"given": "Keith",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Donohoe",
"given": "Katie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brunk",
"given": "Crystal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hernandez",
"given": "Geraldine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jacobucci",
"given": "Tina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lazosky",
"given": "Lynda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mann",
"given": "Puneet",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Raval",
"given": "Geeta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zampieri",
"given": "Ligia Araujo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sekhon",
"given": "Mypinder",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wright",
"given": "Alissa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "James",
"given": "Nicola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chang",
"given": "Gaby",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Roy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Deol",
"given": "Kanwal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gantioqui",
"given": "Jorell",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Larsen",
"given": "Elyse",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ramdin",
"given": "Namita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roche",
"given": "Margaret",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rosinski",
"given": "Kristin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sham",
"given": "Lawrence",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Storms",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gillrie",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mahe",
"given": "Etienne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Suryanarayan",
"given": "Deepa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ugarte-Torres",
"given": "Alejandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Robinson",
"given": "Traci",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gibbs",
"given": "Mitchell",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hewsgirard",
"given": "Julia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Holmes",
"given": "Marnie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McCarthy",
"given": "Joanna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ody",
"given": "Meagan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Doucette",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sligl",
"given": "Wendy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sonpar",
"given": "Ashlesah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Robertson",
"given": "Kimberley",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Narayan",
"given": "Jeffrey",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ravindran",
"given": "Leka",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stewart",
"given": "Breanne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zapernick",
"given": "Lori",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Stephen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sy",
"given": "Eric",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wong",
"given": "Alexander",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gryzb",
"given": "Karolina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Craddock",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fuchs",
"given": "Dennaye",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Myrah",
"given": "Danielle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sunny",
"given": "Sana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harding",
"given": "Sheila Rutledge",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kogilwaimath",
"given": "Siddarth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hodgson",
"given": "Nancy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Johnson",
"given": "Dawn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meier",
"given": "Simona",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thomson",
"given": "Kim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Heendeniya",
"given": "Amila",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Houston",
"given": "Brett",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kenyan",
"given": "Yoav",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lother",
"given": "Sylvain",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Olafson",
"given": "Kendiss",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rush",
"given": "Barret",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wuerz",
"given": "Terry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Solvason",
"given": "Dayna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Albensi",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alias",
"given": "Soumya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Choi",
"given": "Nora",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Curtis",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hutmacher",
"given": "Maureen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kashani",
"given": "Hessam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lane",
"given": "Debra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marten",
"given": "Nicole",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pronyk-Ward",
"given": "Tracey",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rigaux",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Silva",
"given": "Rhonda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tays",
"given": "Quinn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Naidu",
"given": "Renuka",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mathews",
"given": "Jane",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mai",
"given": "Margaret",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Miceli",
"given": "Victoria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Molson",
"given": "Liz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Radhakrishnan",
"given": "Gayathri",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schaefer",
"given": "Linda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haddad",
"given": "Michel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Landry",
"given": "Shannon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chernish",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kruisselbrink",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Theresa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jeromin",
"given": "Jayna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Siddiqui",
"given": "Atif",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Girolametto",
"given": "Carla",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Krokoszynski",
"given": "Kristin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Main",
"given": "Cheryl",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fox-Robichaud",
"given": "Alison",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rochwerg",
"given": "Bram",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kruja",
"given": "Erjona",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ellingham",
"given": "Dana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sampat",
"given": "Disha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tang",
"given": "Ngan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leto",
"given": "Daniela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Karunakaran",
"given": "Meera",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ricciuto",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fusco",
"given": "Kelly",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ghate",
"given": "Taneera",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Robinson",
"given": "Holly",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ball",
"given": "Ian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shalhoub",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Slessarev",
"given": "Marat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Silverman",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nano",
"given": "Eni",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bentall",
"given": "Tracey",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Campbell",
"given": "Eileen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kinney",
"given": "Jeffery",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parvathy",
"given": "Seema",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fera",
"given": "Evridiki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Delfa",
"given": "Anthony La",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nadarajah",
"given": "Jeya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Solow",
"given": "Henry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mendoza",
"given": "Edeliza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Engel",
"given": "Katrina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Monaco",
"given": "Diana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kononow",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Suntharalingam",
"given": "Sutharsan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fralick",
"given": "Mike",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Munshi",
"given": "Laveena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saeed",
"given": "Samia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hajjaj",
"given": "Omar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hsu",
"given": "Elaine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ali",
"given": "Karim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duan",
"given": "Erick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Farjou",
"given": "George",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jenson",
"given": "Lorraine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salib",
"given": "Mary",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patterson",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Anant",
"given": "Swati",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ding",
"given": "Josephine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jomy",
"given": "Jane",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Das",
"given": "Pavani",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Geagea",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ingber",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Owen",
"given": "Elliot",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lostun",
"given": "Alexandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Albano",
"given": "Tashea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chatterjee",
"given": "Antara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Giraldo",
"given": "Manuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hickey",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Ida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Okada",
"given": "Nea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pasquale",
"given": "Nicholas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ponzielli",
"given": "Romina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rahmat",
"given": "Mary",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sabur",
"given": "Shelina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schlag",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aguiar",
"given": "Leonita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Damani",
"given": "Ashmina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hong",
"given": "Suhyoung",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kokabi",
"given": "Mona",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Perkins",
"given": "Carolyn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cowan",
"given": "Juthaporn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Giulivi",
"given": "Tony",
"sequence": "additional"
},
{
"affiliation": [],
"family": "MacFadden",
"given": "Derek",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cyr",
"given": "Joe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pecarskie",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Porteous",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vedder",
"given": "Priscila Ogawa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Watpool",
"given": "Irene",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berardi",
"given": "Phil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bustani",
"given": "Laith",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Graver",
"given": "Alison",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Iyengar",
"given": "Akshai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kisilewicz",
"given": "Magdalena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Majewski",
"given": "Jake",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marovac",
"given": "Misha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Murthy",
"given": "Ruchi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sharma",
"given": "Karan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Walcer",
"given": "Marina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chagla",
"given": "Zain",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cheung",
"given": "Jason",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duan",
"given": "Erick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Clarke",
"given": "France",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Matic",
"given": "Karlo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Giraldo",
"given": "Manuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hickey",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Ida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Okada",
"given": "Nea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pasquale",
"given": "Nicholas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ponzielli",
"given": "Romina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rahmat",
"given": "Mary",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sabur",
"given": "Shelina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schlag",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carpenter",
"given": "Travis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schwartz",
"given": "Kevin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Suthar",
"given": "Paril",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jiwajee",
"given": "Aziz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lindsay",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Malik",
"given": "Aftab",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tse",
"given": "Brandon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Matukas",
"given": "Larissa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ray",
"given": "Joel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bell",
"given": "Shirley",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Krok",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guo",
"given": "Ray",
"sequence": "additional"
},
{
"affiliation": [],
"family": "John",
"given": "Susan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Joshi",
"given": "Vishal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Keen",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lazongas",
"given": "Chris",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ostro",
"given": "Jacqueline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shore",
"given": "Kevin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Jianmin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Choi",
"given": "Jincheol",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nallapati",
"given": "Pujitha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Irwin",
"given": "Tina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Victor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sheldrake",
"given": "Petra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Adhikari",
"given": "Neill",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wunsch",
"given": "Hannah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bailey",
"given": "Jacob",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meirovich",
"given": "Harley",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Colavecchia",
"given": "Connie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kahwash",
"given": "Eiad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sud",
"given": "Sachin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Romano",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Coburn",
"given": "Bryan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Del Sorbo",
"given": "Lorenzo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Granton",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Husain",
"given": "Shahid",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pendergrast",
"given": "Jacob",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sharkawy",
"given": "Abdu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wilcox",
"given": "Liz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saeed",
"given": "Samia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hajjaj",
"given": "Omar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kulikova",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Massin",
"given": "Sophia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kennette",
"given": "Wendy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mazzetti",
"given": "Ian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Naccarato",
"given": "Krista",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Park",
"given": "Grace",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pennetti",
"given": "Alex",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Primeau",
"given": "Corrin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vilag",
"given": "Cathy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lapointe",
"given": "Yves",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lemay",
"given": "Anne-Sophie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duceppe",
"given": "Emmanuelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rioux-Massé",
"given": "Benjamin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tremblay",
"given": "Cécile",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arlotto",
"given": "Pascale",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bouchard",
"given": "Claudia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Matte",
"given": "Stephanie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Messier-Peet",
"given": "Marc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Francoeur",
"given": "Charles-Langis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lauzier",
"given": "François",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leblanc",
"given": "Guillaume",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bellemare",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cloutier",
"given": "Ève",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Costerousse",
"given": "Olivier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chénard",
"given": "Émilie Couillard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daher",
"given": "Rana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daigle",
"given": "Marjorie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grenier",
"given": "Stéphanie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guilbeault",
"given": "Gabrielle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rioux",
"given": "Marie-Pier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "St-Onge",
"given": "Maude",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tremblay",
"given": "Antoine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beaudoin",
"given": "Brian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lanthier",
"given": "Luc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Larrivée",
"given": "Pierre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morin",
"given": "Pierre-Aurèle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carbonneau",
"given": "Élaine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lacasse",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Autmizguine",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boucoiran",
"given": "Isabelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Du Pont-Thibodeau",
"given": "Geneviève",
"sequence": "additional"
},
{
"affiliation": [],
"family": "La Haye",
"given": "Annie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lague",
"given": "Vincent",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Léveillé",
"given": "Karine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Quach-Thanh",
"given": "Caroline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Émériaud",
"given": "Guillaume",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jouvet",
"given": "Philippe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haddad",
"given": "Élie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Turgeon-Provost",
"given": "Camille",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fox",
"given": "Susan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baldé",
"given": "Diaraye",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ménard",
"given": "Lorraine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morissette",
"given": "Suzanne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schnorr-Meloche",
"given": "Miriam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Turcotte",
"given": "Andrée-Anne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vallée",
"given": "Caroline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Castonguay",
"given": "Stéphanie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nguyen",
"given": "Tuyen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rivest",
"given": "Natalie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roussos",
"given": "Marios",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Simoneau",
"given": "Esther",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Belecciu",
"given": "Andreea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bouchard",
"given": "Marie-Hélène",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daviau",
"given": "Eric",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martin",
"given": "Cynthia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sabourin",
"given": "Nicole",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tremblay",
"given": "Solange",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gagné",
"given": "Émilie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gagné",
"given": "Nancy-Lisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Larouche",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Larouche",
"given": "Vanessa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tremblay",
"given": "Véronick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tremblay",
"given": "Vicky",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Blanchette",
"given": "Pierre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Claveau",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lamarre",
"given": "Marianne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tapps",
"given": "Danielle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Albert",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duca",
"given": "Anatolie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leduc",
"given": "Jean-Michel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boudreault-Pedneault",
"given": "Jean-Samuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barsalou",
"given": "Annie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Deschênes-Dion",
"given": "Suzanne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ibrahim",
"given": "Stéphanie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ridyard",
"given": "Stéphanie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rousseau",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahern",
"given": "Stéphane",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arsenault",
"given": "Marie-Pier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dufresne",
"given": "Simon-Frédéric",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mollica",
"given": "Luigina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Hang Ting",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beau",
"given": "Soizic",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beaupré",
"given": "Dominique",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dégarie",
"given": "Marjolaine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Delorme",
"given": "Iris",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Farkas",
"given": "Melissa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gratton",
"given": "Michel-Olivier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guertin",
"given": "Arnaud",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jalbert",
"given": "Guylaine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meilleur",
"given": "Mélanie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Labrecque",
"given": "Charles Ratté",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Santos",
"given": "Élaine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lu",
"given": "Julie Trinh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Auger",
"given": "Julien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lessard",
"given": "Marie-Claude",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mardini",
"given": "Louay",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pesant",
"given": "Yves",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Delves",
"given": "Laurie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Delves",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Denault",
"given": "Sophie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grigorova",
"given": "Sofia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lambert",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Langille",
"given": "Nathalie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Langlois",
"given": "Corinne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rock",
"given": "Caroline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sardin-Laframboise",
"given": "Yannick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Archambault",
"given": "Patrick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bélanger-Pelletier",
"given": "Joannie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duquet-Deblois",
"given": "Estel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dupuis-Picard",
"given": "Vanessa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hamelin",
"given": "Yannick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leduc",
"given": "Samuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Richard",
"given": "Mélanie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fortin",
"given": "Marc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gervais",
"given": "Philippe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boulay",
"given": "Marie-Ève",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ferland",
"given": "Claudine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guertin",
"given": "Jakie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lepage",
"given": "Johane",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roy",
"given": "Annie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Assouline",
"given": "Sarit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Caplan",
"given": "Stephen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kong",
"given": "Ling",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Canticas",
"given": "Christina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mayhew",
"given": "Carley",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ouedraogo",
"given": "Johanne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tep",
"given": "Tévy-Suzy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Batist",
"given": "Gerald",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cheng",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Klein",
"given": "Marina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kronfli",
"given": "Nadine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pelletier",
"given": "Patricia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Qureshi",
"given": "Salman",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vinh",
"given": "Donald",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dziarmaga",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peiris",
"given": "Hansi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Proulx-Boucher",
"given": "Karène",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roger",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rothschild",
"given": "Molly-Ann",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yuen",
"given": "Chung-Yan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barkati",
"given": "Sapha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Routy",
"given": "Jean-Pierre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sinanan-Pelletier",
"given": "Sondra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "LeBlanc",
"given": "Rémi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "St-Hilaire",
"given": "Eve",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thibeault",
"given": "Patrick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morin",
"given": "Karine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Caissie",
"given": "Gilberte",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Collette",
"given": "Jackie Caissie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daigle",
"given": "Line",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daigle",
"given": "Mélissa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gendron",
"given": "Bianca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Godin",
"given": "Nathalie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lapointe",
"given": "Angela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moreau",
"given": "Gabrielle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ouellette-Bernier",
"given": "Lola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rockburn",
"given": "Joanne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sonier-Ferguson",
"given": "Brigitte",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wilson",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "DeSimone",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ellsworth",
"given": "Grant",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fry",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goss",
"given": "Noah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gulick",
"given": "Roy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vaamonde",
"given": "Carlos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wilkin",
"given": "Timothy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arar",
"given": "Celine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berardi",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Dennis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garcia-Miller",
"given": "Cristina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goldbach",
"given": "Arthur",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gripp",
"given": "Lauren",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hayden",
"given": "Danielle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kane",
"given": "Kathleen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Jiamin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marcelin",
"given": "Kinge-Ann",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Megill",
"given": "Christina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nelson",
"given": "Meredith",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paguntalan",
"given": "Ailema",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Raab",
"given": "Gabriel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Resso",
"given": "Gianna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rosario",
"given": "Roxanne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rossen",
"given": "Noah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tamura",
"given": "Shoran",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhao",
"given": "Ethan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goss",
"given": "Cheryl",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kim",
"given": "Young",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Eshan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paul",
"given": "Sonal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Romero",
"given": "Tiffany",
"sequence": "additional"
},
{
"affiliation": [],
"family": "ElBadri",
"given": "Naima",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Flores",
"given": "Lina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sandoval",
"given": "Tricia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kapadia",
"given": "Shashi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vasovic",
"given": "Ljiljana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Griffiths",
"given": "Shanna-Kay",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alvarado",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goudy",
"given": "Fiona",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lewis",
"given": "Melissa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Loizou",
"given": "Marina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Louie",
"given": "Rita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pambrun",
"given": "Chantale",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Torrance",
"given": "Sylvia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Drews",
"given": "Steven",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McManus",
"given": "Janet",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cuevas",
"given": "Oriela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lafresne",
"given": "Wanda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ruoso",
"given": "Patrizia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shin",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Steed",
"given": "Tony",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ward",
"given": "Rachel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Allard",
"given": "Isabelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Germain",
"given": "Marc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Girard",
"given": "Sébastien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parent",
"given": "Éric",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pigeon",
"given": "Claudia-Mireille",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lopes",
"given": "Maria Esther",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pêcego",
"given": "Margarida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rosario",
"given": "Natalia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "da Costa Silva",
"given": "Carlos Alexandre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Oliveira",
"given": "Thais",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lopes",
"given": "Maria Cristina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mateos",
"given": "Sheila",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hall",
"given": "Lucette",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paradiso",
"given": "Sarai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Strauss",
"given": "Donna",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6133-0677",
"affiliation": [],
"authenticated-orcid": false,
"family": "Arnold",
"given": "Donald M.",
"sequence": "additional"
},
{
"affiliation": [],
"name": "the CONCOR-1 Study Group",
"sequence": "additional"
}
],
"container-title": "Nature Medicine",
"container-title-short": "Nat Med",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2021,
9,
9
]
],
"date-time": "2021-09-09T10:06:24Z",
"timestamp": 1631181984000
},
"deposited": {
"date-parts": [
[
2023,
1,
8
]
],
"date-time": "2023-01-08T22:00:59Z",
"timestamp": 1673215259000
},
"indexed": {
"date-parts": [
[
2024,
4,
9
]
],
"date-time": "2024-04-09T19:50:17Z",
"timestamp": 1712692217533
},
"is-referenced-by-count": 197,
"issue": "11",
"issued": {
"date-parts": [
[
2021,
9,
9
]
]
},
"journal-issue": {
"issue": "11",
"published-print": {
"date-parts": [
[
2021,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
9,
9
]
],
"date-time": "2021-09-09T00:00:00Z",
"timestamp": 1631145600000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
9,
9
]
],
"date-time": "2021-09-09T00:00:00Z",
"timestamp": 1631145600000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41591-021-01488-2.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41591-021-01488-2",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41591-021-01488-2.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"page": "2012-2024",
"prefix": "10.1038",
"published": {
"date-parts": [
[
2021,
9,
9
]
]
},
"published-online": {
"date-parts": [
[
2021,
9,
9
]
]
},
"published-print": {
"date-parts": [
[
2021,
11
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1038/s41564-020-00813-8",
"author": "J Seow",
"doi-asserted-by": "publisher",
"first-page": "1598",
"journal-title": "Nat. Microbiol.",
"key": "1488_CR1",
"unstructured": "Seow, J. et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 5, 1598–1607 (2020).",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1503/cmaj.200642",
"author": "N Devasenapathy",
"doi-asserted-by": "publisher",
"first-page": "E745",
"journal-title": "CMAJ",
"key": "1488_CR2",
"unstructured": "Devasenapathy, N. et al. Efficacy and safety of convalescent plasma for severe COVID-19 based on evidence in other severe respiratory viral infections: a systematic review and meta-analysis. CMAJ 192, E745–E755 (2020).",
"volume": "192",
"year": "2020"
},
{
"DOI": "10.1182/blood.2020008903",
"author": "EM Wood",
"doi-asserted-by": "publisher",
"first-page": "1573",
"journal-title": "Blood",
"key": "1488_CR3",
"unstructured": "Wood, E. M., Estcourt, L. J. & McQuilten, Z. K. How should we use convalescent plasma therapies for the management of COVID-19? Blood 137, 1573–1581 (2021).",
"volume": "137",
"year": "2021"
},
{
"DOI": "10.1111/trf.16026",
"author": "D Blackall",
"doi-asserted-by": "publisher",
"first-page": "2203",
"journal-title": "Transfusion",
"key": "1488_CR4",
"unstructured": "Blackall, D. et al. Rapid establishment of a COVID-19 convalescent plasma program in a regional health care delivery network. Transfusion 60, 2203–2209 (2020).",
"volume": "60",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2031304",
"author": "VA Simonovich",
"doi-asserted-by": "publisher",
"first-page": "619",
"journal-title": "N. Engl. J. Med.",
"key": "1488_CR5",
"unstructured": "Simonovich, V. A. et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N. Engl. J. Med. 384, 619–629 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1136/bmj.m3939",
"author": "A Agarwal",
"doi-asserted-by": "publisher",
"first-page": "m3939",
"journal-title": "Brit. Med. J.",
"key": "1488_CR6",
"unstructured": "Agarwal, A. et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). Brit. Med. J. 371, m3939 (2020).",
"volume": "371",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.10044",
"author": "L Li",
"doi-asserted-by": "publisher",
"first-page": "460",
"journal-title": "JAMA",
"key": "1488_CR7",
"unstructured": "Li, L. et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA 324, 460–470 (2020).",
"volume": "324",
"year": "2020"
},
{
"author": "V Piechotta",
"first-page": "CD013600",
"journal-title": "Cochrane Database Syst. Rev.",
"key": "1488_CR8",
"unstructured": "Piechotta, V. et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst. Rev. 5, CD013600 (2021).",
"volume": "5",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2031893",
"author": "MJ Joyner",
"doi-asserted-by": "publisher",
"first-page": "1015",
"journal-title": "N. Engl. J. Med.",
"key": "1488_CR9",
"unstructured": "Joyner, M. J. et al. Convalescent plasma antibody levels and the risk of death from Covid-19. N. Engl. J. Med. 384, 1015–1027 (2021).",
"volume": "384",
"year": "2021"
},
{
"key": "1488_CR10",
"unstructured": "The RECOVERY Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet 397, 2049–2059 (2021)."
},
{
"DOI": "10.1101/2020.11.25.20237883",
"doi-asserted-by": "crossref",
"key": "1488_CR11",
"unstructured": "Ray, Y. et al. Clinical and immunological benefits of convalescent plasma therapy in severe COVID-19: insights from a single center open label randomised control trial. Preprint at https://www.medrxiv.org/content/10.1101/2020.11.25.20237883v1 (2020)."
},
{
"DOI": "10.1038/s41467-021-23469-2",
"author": "A Gharbharan",
"doi-asserted-by": "publisher",
"journal-title": "Nat. Commun.",
"key": "1488_CR12",
"unstructured": "Gharbharan, A. et al. Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection. Nat. Commun. 12, 3189 (2021).",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2033700",
"author": "R Libster",
"doi-asserted-by": "publisher",
"first-page": "610",
"journal-title": "N. Engl. J. Med.",
"key": "1488_CR13",
"unstructured": "Libster, R. et al. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N. Engl. J. Med. 384, 610–618 (2021).",
"volume": "384",
"year": "2021"
},
{
"key": "1488_CR14",
"unstructured": "O’Donnell, M. R. et al. A randomized double-blind controlled trial of convalescent plasma in adults with severe COVID-19. J. Clin. Invest. 131, e150646 (2021)."
},
{
"key": "1488_CR15",
"unstructured": "Avendaño-Solà, C. et al. Convalescent plasma for COVID-19: a multicenter, randomized clinical trial. Preprint at https://www.medrxiv.org/content/10.1101/2020.08.26.20182444v3 (2020)."
},
{
"key": "1488_CR16",
"unstructured": "Estcourt, L. J. Convalescent plasma in critically ill patients with Covid-19. Preprint at https://www.medrxiv.org/content/10.1101/2021.06.11.21258760v1 (2021)."
},
{
"DOI": "10.1097/CCM.0000000000005066",
"doi-asserted-by": "crossref",
"key": "1488_CR17",
"unstructured": "Bennett-Guerrero, E. et al. Severe acute respiratory syndrome coronavirus 2 convalescent plasma versus standard plasma in Coronavirus Disease 2019 infected hospitalized patients in New York: a double-blind randomized trial. Crit. Care Med. 49, 1015–1025 (2021)."
},
{
"key": "1488_CR18",
"unstructured": "Körper, S. et al. High dose convalescent plasma in COVID-19: results from the randomized trial CAPSID. Preprint at https://www.medrxiv.org/content/10.1101/2021.05.10.21256192v1 (2021)."
},
{
"DOI": "10.1038/s41598-021-89444-5",
"author": "M AlQahtani",
"doi-asserted-by": "publisher",
"journal-title": "Sci. Rep.",
"key": "1488_CR19",
"unstructured": "AlQahtani, M. et al. Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease. Sci. Rep. 11, 9927 (2021).",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1080/11101849.2020.1842087",
"author": "O Hamdy Salman",
"doi-asserted-by": "publisher",
"first-page": "264",
"journal-title": "Egypt. J. Anaesth.",
"key": "1488_CR20",
"unstructured": "Hamdy Salman, O. & Ail Mohamed, H. S. Efficacy and safety of transfusing plasma from COVID-19 survivors to COVID-19 victims with severe illness. A double-blinded controlled preliminary study. Egypt. J. Anaesth. 36, 264–272 (2020).",
"volume": "36",
"year": "2020"
},
{
"DOI": "10.1101/2020.10.25.20219337",
"doi-asserted-by": "crossref",
"key": "1488_CR21",
"unstructured": "Bajpai, M. et al. efficacy of convalescent plasma therapy compared to fresh frozen plasma in severely ill COVID-19 patients: a pilot randomized controlled trial. Preprint at https://www.medrxiv.org/content/10.1101/2020.10.25.20219337v1 (2020)."
},
{
"DOI": "10.1016/j.cell.2021.02.026",
"author": "ES Winkler",
"doi-asserted-by": "publisher",
"first-page": "1804",
"journal-title": "Cell",
"key": "1488_CR22",
"unstructured": "Winkler, E. S. et al. Human neutralizing antibodies against SARS-CoV-2 require intact Fc effector functions for optimal therapeutic protection. Cell 184, 1804–1820 (2021).",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2021.03.029",
"author": "N Suryadevara",
"doi-asserted-by": "publisher",
"first-page": "2316",
"journal-title": "Cell",
"key": "1488_CR23",
"unstructured": "Suryadevara, N. et al. Neutralizing and protective human monoclonal antibodies recognizing the N-terminal domain of the SARS-CoV-2 spike protein. Cell 184, 2316–2331 (2021).",
"volume": "184",
"year": "2021"
},
{
"key": "1488_CR24",
"unstructured": "Ullah, I. et al. Live imaging of SARS-CoV-2 infection in mice reveals neutralizing antibodies require Fc function for optimal efficacy. Preprint at https://www.biorxiv.org/content/10.1101/2021.03.22.436337v1.full (2021)."
},
{
"DOI": "10.1084/jem.20201993",
"doi-asserted-by": "crossref",
"key": "1488_CR25",
"unstructured": "Schafer, A. et al. Antibody potency, effector function, and combinations in protection and therapy for SARS-CoV-2 infection in vivo. J. Exp. Med. 218, e20201993 (2021)."
},
{
"DOI": "10.1101/2021.03.18.21253907",
"doi-asserted-by": "crossref",
"key": "1488_CR26",
"unstructured": "Brunet-Ratnasingham, E. A. S. et al. Integrated immunovirological profiling validates plasma SARS-CoV-2 RNA as an early predictor of COVID-19 mortality. Preprint at https://www.medrxiv.org/content/10.1101/2021.03.18.21253907v1 (2021)."
},
{
"DOI": "10.1016/j.cell.2020.10.052",
"author": "T Zohar",
"doi-asserted-by": "publisher",
"first-page": "1508",
"journal-title": "Cell",
"key": "1488_CR27",
"unstructured": "Zohar, T. et al. Compromised humoral functional evolution tracks with SARS-CoV-2 mortality. Cell 183, 1508–1519 (2020).",
"volume": "183",
"year": "2020"
},
{
"DOI": "10.1056/NEJMc2033787",
"author": "A Casadevall",
"doi-asserted-by": "publisher",
"first-page": "189",
"journal-title": "N. Engl. J. Med.",
"key": "1488_CR28",
"unstructured": "Casadevall, A., Joyner, M. J. & Pirofski, L. A. Neutralizing antibody LY-CoV555 for outpatient Covid-19. N. Engl. J. Med. 384, 189 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa1113425",
"author": "BF Haynes",
"doi-asserted-by": "publisher",
"first-page": "1275",
"journal-title": "N. Engl. J. Med.",
"key": "1488_CR29",
"unstructured": "Haynes, B. F. et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 366, 1275–1286 (2012).",
"volume": "366",
"year": "2012"
},
{
"DOI": "10.1073/pnas.1301456110",
"author": "GD Tomaras",
"doi-asserted-by": "publisher",
"first-page": "9019",
"journal-title": "Proc. Natl Acad. Sci. USA",
"key": "1488_CR30",
"unstructured": "Tomaras, G. D. et al. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc. Natl Acad. Sci. USA 110, 9019–9024 (2013).",
"volume": "110",
"year": "2013"
},
{
"DOI": "10.4049/jimmunol.170.7.3621",
"author": "CP Taborda",
"doi-asserted-by": "publisher",
"first-page": "3621",
"journal-title": "J. Immunol.",
"key": "1488_CR31",
"unstructured": "Taborda, C. P., Rivera, J., Zaragoza, O. & Casadevall, A. More is not necessarily better: prozone-like effects in passive immunization with IgG. J. Immunol. 170, 3621–3630 (2003).",
"volume": "170",
"year": "2003"
},
{
"DOI": "10.1016/j.ijid.2021.06.034",
"author": "FW Hamilton",
"doi-asserted-by": "publisher",
"first-page": "114",
"journal-title": "Int. J. Infect. Dis.",
"key": "1488_CR32",
"unstructured": "Hamilton, F. W., Lee, T., Arnold, D. T., Lilford, R. & Hemming, K. Is convalescent plasma futile in COVID-19? A Bayesian re-analysis of the RECOVERY randomized controlled trial. Int. J. Infect. Dis. 109, 114–117 (2021).",
"volume": "109",
"year": "2021"
},
{
"DOI": "10.1111/j.1537-2995.2011.03247.x",
"author": "C Narick",
"doi-asserted-by": "publisher",
"first-page": "160",
"journal-title": "Transfusion",
"key": "1488_CR33",
"unstructured": "Narick, C., Triulzi, D. J. & Yazer, M. H. Transfusion-associated circulatory overload after plasma transfusion. Transfusion 52, 160–165 (2012).",
"volume": "52",
"year": "2012"
},
{
"DOI": "10.1186/s13063-021-05235-3",
"author": "P Begin",
"doi-asserted-by": "publisher",
"journal-title": "Trials",
"key": "1488_CR34",
"unstructured": "Begin, P. et al. Convalescent plasma for adults with acute COVID-19 respiratory illness (CONCOR-1): study protocol for an international, multicentre, randomized, open-label trial. Trials 22, 323 (2021).",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1016/j.jbi.2008.08.010",
"author": "PA Harris",
"doi-asserted-by": "publisher",
"first-page": "377",
"journal-title": "J. Biomed. Inform.",
"key": "1488_CR35",
"unstructured": "Harris, P. A. et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 42, 377–381 (2009).",
"volume": "42",
"year": "2009"
},
{
"DOI": "10.1111/trf.15910",
"author": "A Budhai",
"doi-asserted-by": "publisher",
"first-page": "1348",
"journal-title": "Transfusion",
"key": "1488_CR36",
"unstructured": "Budhai, A. et al. How did we rapidly implement a convalescent plasma program? Transfusion 60, 1348–1355 (2020).",
"volume": "60",
"year": "2020"
},
{
"DOI": "10.1172/jci.insight.142362",
"doi-asserted-by": "crossref",
"key": "1488_CR37",
"unstructured": "Abe, K. T. et al. A simple protein-based surrogate neutralization assay for SARS-CoV-2. JCI Insight. 5, e142362 (2020)."
},
{
"DOI": "10.1002/cpmc.105",
"author": "EJ Mendoza",
"doi-asserted-by": "crossref",
"first-page": "ecpmc105",
"journal-title": "Curr. Protoc. Microbiol.",
"key": "1488_CR38",
"unstructured": "Mendoza, E. J., Manguiat, K., Wood, H. & Drebot, M. Two detailed plaque assay protocols for the quantification of infectious SARS-CoV-2. Curr. Protoc. Microbiol. 57, ecpmc105 (2020).",
"volume": "57",
"year": "2020"
},
{
"DOI": "10.1101/2020.07.09.194639",
"doi-asserted-by": "crossref",
"key": "1488_CR39",
"unstructured": "Beaudoin-Bussieres, G. et al. Decline of humoral responses against SARS-CoV-2 spike in convalescent individuals. Preprint at https://www.biorxiv.org/content/10.1101/2020.07.09.194639v1 (2020)."
},
{
"DOI": "10.1182/blood.2020008367",
"author": "J Perreault",
"doi-asserted-by": "publisher",
"first-page": "2588",
"journal-title": "Blood",
"key": "1488_CR40",
"unstructured": "Perreault, J. et al. Waning of SARS-CoV-2 RBD antibodies in longitudinal convalescent plasma samples within 4 months after symptom onset. Blood 136, 2588–2591 (2020).",
"volume": "136",
"year": "2020"
},
{
"DOI": "10.1111/trf.16318",
"doi-asserted-by": "crossref",
"key": "1488_CR41",
"unstructured": "Anand, S. P. et al. High-throughput detection of antibodies targeting the SARS-CoV-2 spike in longitudinal convalescent plasma samples. Transfusion 61, 1377–1382 (2021)."
},
{
"DOI": "10.1101/2021.03.18.435972",
"doi-asserted-by": "crossref",
"key": "1488_CR42",
"unstructured": "Tauzin, A. N. M. et al. A single BNT162b2 mRNA dose elicits antibodies with Fc-mediated effector functions and boost pre-existing humoral and T cell responses. Preprint at https://www.biorxiv.org/content/10.1101/2021.03.18.435972v1 (2021)."
},
{
"DOI": "10.1016/j.xcrm.2021.100290",
"doi-asserted-by": "crossref",
"key": "1488_CR43",
"unstructured": "Anand, S. P. et al. Longitudinal analysis of humoral immunity against SARS-CoV-2 spike in convalescent individuals up to eight months post-symptom onset. Cell Rep. Med. 2, 100290 (2021)."
},
{
"DOI": "10.1201/9781315370279",
"doi-asserted-by": "crossref",
"key": "1488_CR44",
"unstructured": "Wood, S. N. Generalized Additive Models: An Introduction with R, Second Edition (Taylor and Francis, 2017)."
},
{
"DOI": "10.2307/2530245",
"author": "PC O’Brien",
"doi-asserted-by": "publisher",
"first-page": "549",
"journal-title": "Biometrics",
"key": "1488_CR45",
"unstructured": "O’Brien, P. C. & Fleming, T. R. A multiple testing procedure for clinical trials. Biometrics 35, 549–556 (1979).",
"volume": "35",
"year": "1979"
},
{
"key": "1488_CR46",
"unstructured": "R Development Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2010)."
},
{
"DOI": "10.1111/j.1467-9868.2010.00749.x",
"author": "SN Wood",
"doi-asserted-by": "publisher",
"first-page": "3",
"journal-title": "J. R. Stat. Soc. Ser. B (Methodol.)",
"key": "1488_CR47",
"unstructured": "Wood, S. N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B (Methodol.) 73, 3–36 (2011).",
"volume": "73",
"year": "2011"
}
],
"reference-count": 47,
"references-count": 47,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41591-021-01488-2"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Biochemistry, Genetics and Molecular Biology",
"General Medicine"
],
"subtitle": [],
"title": "Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "27"
}