Pyrimidine inhibitors synergize with nucleoside analogues to block SARS-CoV-2
et al., Nature, doi:10.1038/s41586-022-04482-x, Feb 2022
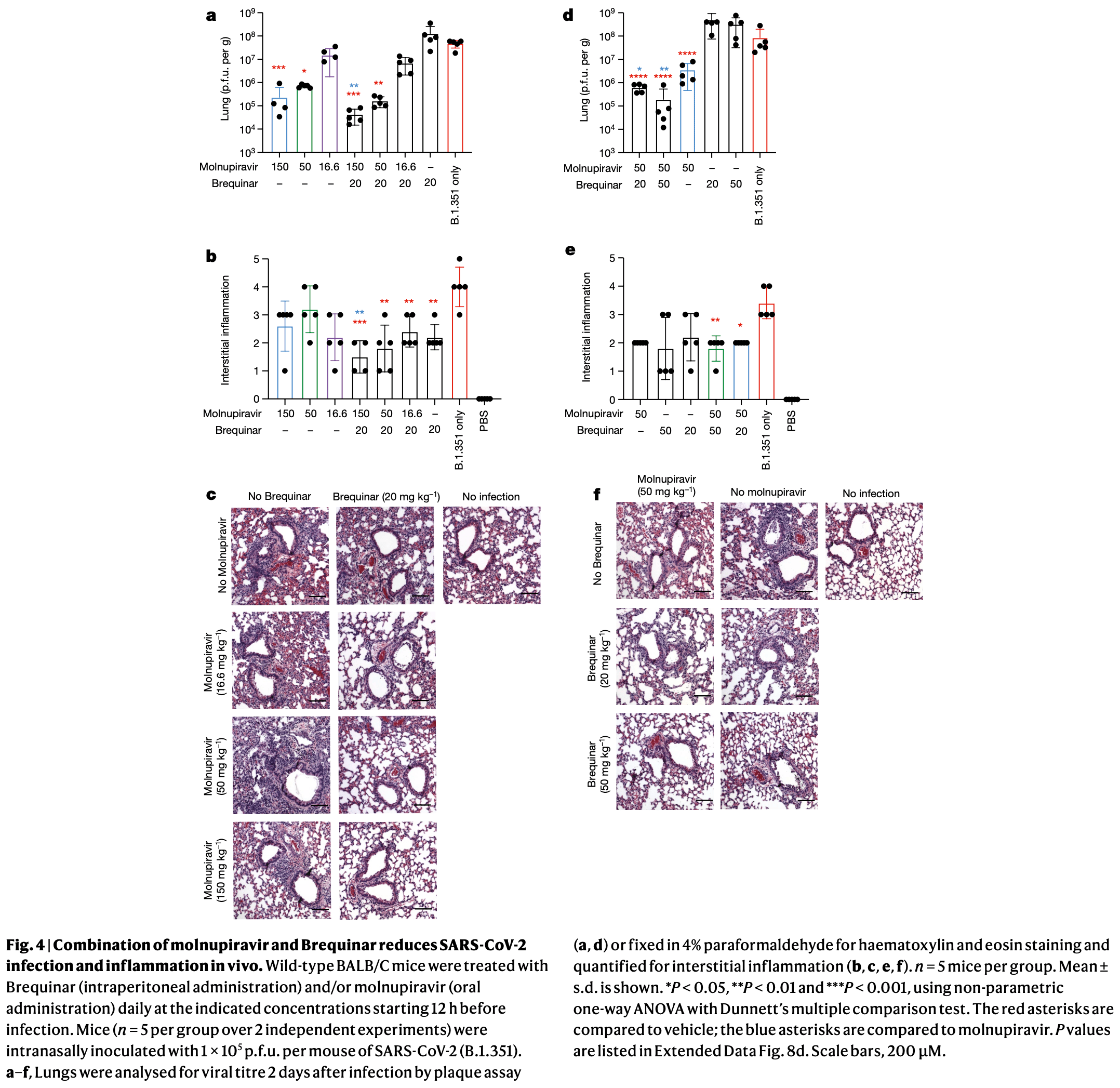
In vitro and mouse study showing synergistic antiviral effects when combining pyrimidine biosynthesis inhibitors with antiviral nucleoside analogues against SARS-CoV-2. Authors screened 18 thousand drugs and validated 122 with antiviral activity against SARS-CoV-2 in human respiratory cells, including remdesivir, molnupiravir, camostat, nafamostat, and artesunate. Pyrimidine biosynthesis inhibitors such as brequinar and BAY-2402234 (DHODH inhibitors) showed modest antiviral activity alone but demonstrated strong synergy when combined with molnupiravir or remdesivir. In mouse models infected with the SARS-CoV-2 Beta variant, the combination of molnupiravir with brequinar showed significantly reduced viral titers (up to 4-log reduction) and decreased lung inflammation compared to either drug alone. The synergistic effect was observed in multiple cell types and was effective against multiple SARS-CoV-2 variants.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
Schultz et al., 7 Feb 2022, USA, peer-reviewed, 22 authors.
Contact: dschultz@pennmedicine.upenn.edu, mfrieman@som.umaryland.edu, cherrys@pennmedicine.upenn.edu.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Abstract: Article
Pyrimidine inhibitors synergize with
nucleoside analogues to block SARS-CoV-2
https://doi.org/10.1038/s41586-022-04482-x
Received: 22 October 2021
Accepted: 26 January 2022
Published online: 7 February 2022
David C. Schultz1 ✉, Robert M. Johnson2,8, Kasirajan Ayyanathan3,8, Jesse Miller3,8,
Kanupriya Whig1, Brinda Kamalia1, Mark Dittmar3, Stuart Weston2, Holly L. Hammond2,
Carly Dillen2, Jeremy Ardanuy2, Louis Taylor2, Jae Seung Lee3, Minghua Li3, Emily Lee4,
Clarissa Shoffler5, Christopher Petucci5, Samuel Constant6, Marc Ferrer4,
Christoph A. Thaiss7, Matthew B. Frieman2 ✉ & Sara Cherry1,3,7 ✉
Check for updates
The SARS-CoV-2 virus has infected more than 261 million people and has led to more
than 5 million deaths in the past year and a half1 (https://www.who.org/). Individuals
with SARS-CoV-2 infection typically develop mild-to-severe flu-like symptoms,
whereas infection of a subset of individuals leads to severe-to-fatal clinical outcomes2.
Although vaccines have been rapidly developed to combat SARS-CoV-2, there has
been a dearth of antiviral therapeutics. There is an urgent need for therapeutics,
which has been amplified by the emerging threats of variants that may evade vaccines.
Large-scale efforts are underway to identify antiviral drugs. Here we screened
approximately 18,000 drugs for antiviral activity using live virus infection in human
respiratory cells and validated 122 drugs with antiviral activity and selectivity against
SARS-CoV-2. Among these candidates are 16 nucleoside analogues, the largest
category of clinically used antivirals. This included the antivirals remdesivir and
molnupiravir, which have been approved for use in COVID-19. RNA viruses rely on a
high supply of nucleoside triphosphates from the host to efficiently replicate, and we
identified a panel of host nucleoside biosynthesis inhibitors as antiviral. Moreover, we
found that combining pyrimidine biosynthesis inhibitors with antiviral nucleoside
analogues synergistically inhibits SARS-CoV-2 infection in vitro and in vivo against
emerging strains of SARS-CoV-2, suggesting a clinical path forward.
SARS-CoV-2 is a coronavirus, which is a family of single-stranded
positive-sense RNA viruses, at least seven of which infect humans.
RNA viruses including coronaviruses replicate using a virally encoded
RNA-dependent RNA polymerase (RdRp), and nucleoside analogues,
which are incorporated by the RdRp into the growing viral RNA chain,
are a large class of approved direct-acting antivirals3. Depending on
the analogue, incorporation can lead to chain termination or mutagenesis, ultimately inhibiting viral replication4. RdRps have conserved
structures, and thus nucleoside analogues can show broad activity
across related and unrelated viruses5,6. Therefore, repurposing efforts
have identified nucleoside analogues that are active against newly
emerging viruses, and such efforts have discovered that the nucleoside
analogue remdesivir inhibits SARS-CoV-2 replication, becoming the
first approved antiviral therapeutic against this novel coronavirus7,8.
As all viruses, including SARS-CoV-2, are dependent on diverse
cellular factors and metabolic products for their replication, the
identification of host-directed antivirals also shows promise. In particular, host nucleoside biogenesis is required for viral replication as
RNA viruses require high levels of nucleoside triphosphates for their
growth. Widespread efforts are underway to identify essential..
DOI record:
{
"DOI": "10.1038/s41586-022-04482-x",
"ISSN": [
"0028-0836",
"1476-4687"
],
"URL": "http://dx.doi.org/10.1038/s41586-022-04482-x",
"alternative-id": [
"4482"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "22 October 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "26 January 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "7 February 2022"
},
{
"label": "Free to read",
"name": "free",
"value": "This content has been made available to all."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-7890-8815",
"affiliation": [],
"authenticated-orcid": false,
"family": "Schultz",
"given": "David C.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-1976-7688",
"affiliation": [],
"authenticated-orcid": false,
"family": "Johnson",
"given": "Robert M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ayyanathan",
"given": "Kasirajan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7990-4912",
"affiliation": [],
"authenticated-orcid": false,
"family": "Miller",
"given": "Jesse",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9114-4365",
"affiliation": [],
"authenticated-orcid": false,
"family": "Whig",
"given": "Kanupriya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kamalia",
"given": "Brinda",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2884-5630",
"affiliation": [],
"authenticated-orcid": false,
"family": "Dittmar",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Weston",
"given": "Stuart",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hammond",
"given": "Holly L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dillen",
"given": "Carly",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ardanuy",
"given": "Jeremy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Taylor",
"given": "Louis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Jae Seung",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Minghua",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Emily",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9064-2378",
"affiliation": [],
"authenticated-orcid": false,
"family": "Shoffler",
"given": "Clarissa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Petucci",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Constant",
"given": "Samuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ferrer",
"given": "Marc",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8226-7718",
"affiliation": [],
"authenticated-orcid": false,
"family": "Thaiss",
"given": "Christoph A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0107-0775",
"affiliation": [],
"authenticated-orcid": false,
"family": "Frieman",
"given": "Matthew B.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3956-6610",
"affiliation": [],
"authenticated-orcid": false,
"family": "Cherry",
"given": "Sara",
"sequence": "additional"
}
],
"container-title": [
"Nature"
],
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
2,
7
]
],
"date-time": "2022-02-07T11:15:53Z",
"timestamp": 1644232553000
},
"deposited": {
"date-parts": [
[
2022,
2,
7
]
],
"date-time": "2022-02-07T12:01:04Z",
"timestamp": 1644235264000
},
"indexed": {
"date-parts": [
[
2022,
2,
7
]
],
"date-time": "2022-02-07T12:42:44Z",
"timestamp": 1644237764036
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "0028-0836"
},
{
"type": "electronic",
"value": "1476-4687"
}
],
"issued": {
"date-parts": [
[
2022,
2,
7
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.springer.com/tdm",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
7
]
],
"date-time": "2022-02-07T00:00:00Z",
"timestamp": 1644192000000
}
},
{
"URL": "https://www.springer.com/tdm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
2,
7
]
],
"date-time": "2022-02-07T00:00:00Z",
"timestamp": 1644192000000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41586-022-04482-x.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41586-022-04482-x",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41586-022-04482-x.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2022,
2,
7
]
]
},
"published-online": {
"date-parts": [
[
2022,
2,
7
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [
"Nature"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": [
"Pyrimidine inhibitors synergize with nucleoside analogues to block SARS-CoV-2"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy"
}