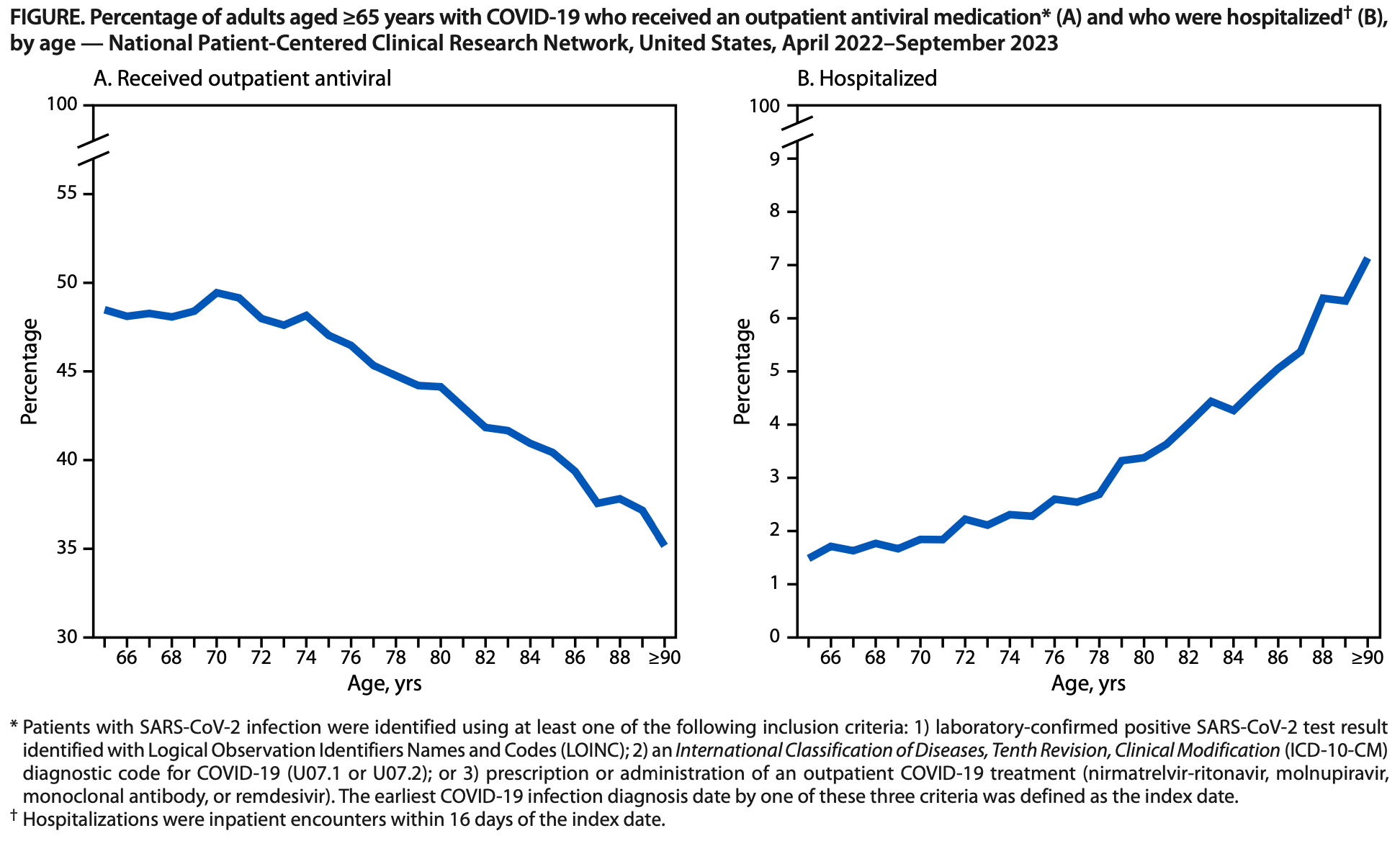
Differences in COVID-19 Outpatient Antiviral Treatment Among Adults Aged ≥65 Years by Age Group — National Patient-Centered Clinical Research Network, United States, April 2022–September 2023
et al., MMWR. Morbidity and Mortality Weekly Report, doi:10.15585/mmwr.mm7339a3, Oct 2024
Retrospective 393,390 COVID-19 patients aged ≥65 years showing underutilization of antiviral treatment, with lower use for older patients at higher risk for severe outcomes.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
Study covers molnupiravir and paxlovid.
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
Quinlan et al., 3 Oct 2024, retrospective, USA, peer-reviewed, 17 authors, study period April 2022 - September 2023.
Differences in COVID-19 Outpatient Antiviral Treatment Among Adults Aged ≥65 Years by Age Group -National Patient-Centered Clinical Research Network, United States
Adults aged ≥65 years experience the highest risk for COVID-19-related hospitalization and death, with risk increasing with increasing age; outpatient antiviral treatment reduces the risk for these severe outcomes. Despite the proven benefit of COVID-19 antiviral treatment, information on differences in use among older adults with COVID-19 by age group is limited. Nonhospitalized patients aged ≥65 years with COVID-19 during April 2022-September 2023 were identified from the National Patient-Centered Clinical Research Network. Differences in use of antiviral treatment among patients aged 65-74, 75-89, and ≥90 years were assessed. Multivariable logistic regression was used to estimate the association between age and nonreceipt of antiviral treatment. Among 393,390 persons aged ≥65 years, 45.9% received outpatient COVID-19 antivirals, including 48.4%, 43.5%, and 35.2% among those aged 65-75, 76-89, and ≥90 years, respectively. Patients aged 75-89 and ≥90 years had 1.17 (95% CI = 1.15-1.19) and 1.54 (95% CI = 1.49-1.61) times the adjusted odds of being untreated, respectively, compared with those aged 65-74 years. Among 12,543 patients with severe outcomes, 2,648 (21.1%) had received an outpatient COVID-19 antiviral medication, compared with 177,874 (46.7%) of 380,847 patients without severe outcomes. Antiviral use is underutilized among adults ≥65 years; the oldest adults are least likely to receive treatment. To prevent COVID-19-associated morbidity and mortality, increased use of COVID-19 antiviral medications among older adults is needed. * PCORnet is a national network that facilitates access to health care data and interoperability through the use of a common data model across participating health care systems. The PCORnet Common Data Model includes demographic characteristics, diagnoses, prescriptions, procedures, and laboratory test results, among other inpatient and outpatient elements, from approximately 30 million patients. https://pcornet.org/data † LOINC is a code system that includes identifiers, names, and codes for clinical and laboratory observations, health care screening instruments, and document types. https://loinc.org § https://www.cdc.gov/nchs/icd/icd-10-cm/files.html ¶ Nirmatrelvir-ritonavir and molnupiravir are taken orally; remdesivir and monoclonal antibodies are administered intravenously. The only monoclonal antibody available for outpatient treatment during the study period was bebtelovimab. However, on November 30, 2022, the Food and Drug Administration announced that bebtelovimab was not authorized because it was not expected to neutralize the variants in circulation at that time.
found that prevalence of receipt of antivirals decreased progressively and substantially with increasing age in persons aged 65 to ≥90 years, after controlling for the number of underlying medical conditions and other demographic factors. Several real-world studies, including those conducted since the emergence of SARS-CoV-2 Omicron variant in January 2022, have demonstrated that COVID-19 antivirals are effective in preventing hospitalization and death (3). Because older age is a strong risk factor for severe COVID-19-associated outcomes, and COVID-19 hospitalizations continue to disproportionately affect older patients (1-4), treatment of COVID-19, including cases in older adults, is critical to the prevention of severe outcomes. Among older patients, frequent self-reported reasons for nonuse of antivirals include the presence of mild signs and symptoms, lack of awareness of eligibility, and absence of a provider recommendation (8) . Other potential barriers to treatment among older patients include delays in seeking treatment after symptom onset and missing the treatment window (5-7 days after symptom onset) (8) . Challenges to COVID-19 antiviral use include obtaining testing (9) , acquiring an antiviral prescription after receiving a positive SARS-CoV-2 test result, and accessing treatment, with each step potentially requiring a separate visit to a health care facility. Older age is associated with increasing numbers of comorbidities and potentially related medications,..
References
Ahmad, Cisewski, Xu, Anderson, COVID-19 mortality update-United States, 2022, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7218a4
Benchimol-Elkaim, Dryden-Peterson, Miller, Koh, Geller, Oral antiviral therapy utilization among adults with recent COVID-19 in the United States, J Gen Intern Med, doi:10.1007/s11606-023-08106-6
Boehmer, Koumans, Skillen, Racial and ethnic disparities in outpatient treatment of COVID-19-United States, January-July 2022, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7143a2
Dryden-Peterson, Kim, Kim, Nirmatrelvir plus ritonavir for early COVID-19 in a large US health system: a population-based cohort study, Ann Intern Med, doi:10.7326/M22-2141
Smith, Lambrou, Patel, SARS-CoV-2 rebound with and without use of COVID-19 oral antivirals, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7251a1
Sun, Hua, Qiu, Brown, Determinants of COVID-19 testing among late middle-aged and older adults: applying the health belief model, Aging Health Res, doi:10.1016/j.ahr.2022.100066
Sun, Rogers, Her, Adaptation and validation of the combined comorbidity score for ICD-10-CM, Med Care, doi:10.1097/MLR.0000000000000824
Taylor, Patel, Pham, COVID-19-associated hospitalizations among U.S. adults aged ≥18 years-COVID-NET, 12 states, October 2023, MMWR Morb Mortal Wkly Rep
Wilcock, Kissler, Mehrotra, Clinical risk and outpatient therapy utilization for COVID-19 in the Medicare population, JAMA Health Forum, doi:10.1001/jamahealthforum.2023.5044
DOI record:
{
"DOI": "10.15585/mmwr.mm7339a3",
"ISSN": [
"0149-2195",
"1545-861X"
],
"URL": "http://dx.doi.org/10.15585/mmwr.mm7339a3",
"author": [
{
"affiliation": [],
"family": "Quinlan",
"given": "Claire M.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Shah",
"given": "Melisa M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "DeSantis",
"given": "Carol E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bertumen",
"given": "J. Bradford",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Draper",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahmad",
"given": "Faraz S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arnold",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mayer",
"given": "Kenneth H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carton",
"given": "Thomas W.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cowell",
"given": "Lindsay G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smith",
"given": "Samantha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saydah",
"given": "Sharon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jones",
"given": "Jefferson M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Pragna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hagen",
"given": "Melissa Briggs",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Block",
"given": "Jason",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Koumans",
"given": "Emily H.",
"sequence": "additional"
}
],
"container-title": "MMWR. Morbidity and Mortality Weekly Report",
"container-title-short": "MMWR Morb. Mortal. Wkly. Rep.",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
10,
3
]
],
"date-time": "2024-10-03T15:25:21Z",
"timestamp": 1727969121000
},
"deposited": {
"date-parts": [
[
2024,
10,
3
]
],
"date-time": "2024-10-03T15:25:24Z",
"timestamp": 1727969124000
},
"indexed": {
"date-parts": [
[
2024,
10,
4
]
],
"date-time": "2024-10-04T04:21:45Z",
"timestamp": 1728015705961
},
"is-referenced-by-count": 0,
"issue": "39",
"issued": {
"date-parts": [
[
2024,
10,
3
]
]
},
"journal-issue": {
"issue": "39",
"published-online": {
"date-parts": [
[
2024,
10,
3
]
]
}
},
"member": "6224",
"original-title": [],
"page": "876-882",
"prefix": "10.15585",
"published": {
"date-parts": [
[
2024,
10,
3
]
]
},
"published-online": {
"date-parts": [
[
2024,
10,
3
]
]
},
"published-other": {
"date-parts": [
[
2024,
10,
3
]
]
},
"publisher": "Centers for Disease Control MMWR Office",
"reference": [
{
"DOI": "10.15585/mmwr.mm7240a3",
"article-title": "COVID-19–associated hospitalizations among U.S. adults aged ≥18 years—COVID-NET, 12 states, October 2023–April 2024",
"author": "Taylor",
"doi-asserted-by": "publisher",
"first-page": "869",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "key-10.15585/mmwr.mm7339a3-202410031124-R1",
"volume": "73",
"year": "2024"
},
{
"key": "key-10.15585/mmwr.mm7339a3-202410031124-R2",
"unstructured": "CDC. COVID-19: underlying conditions and the higher risk for severe COVID-19. Atlanta, GA: US Department of Health and Human Services, CDC; 2023. https://www.cdc.gov/covid/hcp/clinical-care/underlying-conditions.html#:~:text=Having%20multiple%20conditions%20was%20also,of%20patients)%20increased%20with%20age"
},
{
"DOI": "10.7326/M22-2141",
"article-title": "Nirmatrelvir plus ritonavir for early COVID-19 in a large US health system: a population-based cohort study.",
"author": "Dryden-Peterson",
"doi-asserted-by": "publisher",
"first-page": "77",
"journal-title": "Ann Intern Med",
"key": "key-10.15585/mmwr.mm7339a3-202410031124-R3",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.15585/mmwr.mm7218a4",
"article-title": "COVID-19 mortality update—United States, 2022.",
"author": "Ahmad",
"doi-asserted-by": "publisher",
"first-page": "493",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "key-10.15585/mmwr.mm7339a3-202410031124-R4",
"volume": "72",
"year": "2023"
},
{
"DOI": "10.1001/jamahealthforum.2023.5044",
"article-title": "Clinical risk and outpatient therapy utilization for COVID-19 in the Medicare population.",
"author": "Wilcock",
"doi-asserted-by": "publisher",
"first-page": "e235044",
"journal-title": "JAMA Health Forum",
"key": "key-10.15585/mmwr.mm7339a3-202410031124-R5",
"volume": "5",
"year": "2024"
},
{
"DOI": "10.15585/mmwr.mm7143a2",
"article-title": "Racial and ethnic disparities in outpatient treatment of COVID-19—United States, January–July 2022.",
"author": "Boehmer",
"doi-asserted-by": "publisher",
"first-page": "1359",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "key-10.15585/mmwr.mm7339a3-202410031124-R6",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1097/MLR.0000000000000824",
"article-title": "Adaptation and validation of the combined comorbidity score for ICD-10-CM.",
"author": "Sun",
"doi-asserted-by": "publisher",
"first-page": "1046",
"journal-title": "Med Care",
"key": "key-10.15585/mmwr.mm7339a3-202410031124-R7",
"volume": "55",
"year": "2017"
},
{
"DOI": "10.1007/s11606-023-08106-6",
"article-title": "Oral antiviral therapy utilization among adults with recent COVID-19 in the United States.",
"author": "Benchimol-Elkaim",
"doi-asserted-by": "publisher",
"first-page": "1717",
"journal-title": "J Gen Intern Med",
"key": "key-10.15585/mmwr.mm7339a3-202410031124-R8",
"volume": "38",
"year": "2023"
},
{
"DOI": "10.1016/j.ahr.2022.100066",
"article-title": "Determinants of COVID-19 testing among late middle-aged and older adults: applying the health belief model.",
"author": "Sun",
"doi-asserted-by": "publisher",
"first-page": "100066",
"journal-title": "Aging Health Res",
"key": "key-10.15585/mmwr.mm7339a3-202410031124-R9",
"volume": "2",
"year": "2022"
},
{
"DOI": "10.15585/mmwr.mm7251a1",
"article-title": "SARS-CoV-2 rebound with and without use of COVID-19 oral antivirals.",
"author": "Smith",
"doi-asserted-by": "publisher",
"first-page": "1357",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "key-10.15585/mmwr.mm7339a3-202410031124-R10",
"volume": "72",
"year": "2023"
}
],
"reference-count": 10,
"references-count": 10,
"relation": {},
"resource": {
"primary": {
"URL": "http://www.cdc.gov/mmwr/volumes/73/wr/mm7339a3.htm?s_cid=mm7339a3_w"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Differences in COVID-19 Outpatient Antiviral Treatment Among Adults Aged ≥65 Years by Age Group — National Patient-Centered Clinical Research Network, United States, April 2022–September 2023",
"type": "journal-article",
"volume": "73"
}