Comparison of treatment of COVID-19 with inhaled bromhexine, higher doses of colchicine and hymecromone with WHO-recommended paxlovid, molnupiravir, remdesivir, anti-IL-6 receptor antibodies and baricitinib
, V., Pharmacia, doi:10.3897/pharmacia.70.e112550, Oct 2023
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Review of treatment of COVID-19 with inhaled bromhexine, higher doses of colchicine and hymecromone compared to WHO-recommended paxlovid, molnupiravir, remdesivir, anti-IL-6 receptor antibodies, and baricitinib. The author argues that the main cause of death in COVID-19 is cytokine storm caused by NLRP3 inflammasome hyperreaction, and proposes a therapeutic strategy based on inhibiting SARS-CoV-2 cell entry with bromhexine and inhibiting the NLRP3 inflammasome with higher doses of colchicine. Author reports that in 452 inpatients treated with higher colchicine doses, mortality was about 5 times lower than the control group, and argues the doses used are safe and that attending physicians must be familiar with potential side effects and drug interactions. Author also includes the hyaluronan synthesis inhibitor hymecromone in their inpatient treatment regimen. Overall, the author concludes that treatment with inhaled bromhexine, colchicine and hymecromone is more effective, safer and cheaper than the WHO-recommended drugs.
See Mitev et al. for another review covering colchicine for COVID-19.
Review covers TMPRSS2 inhibitors, bromhexine, and colchicine.
Mitev et al., 20 Oct 2023, peer-reviewed, 1 author.
Contact: vmitev@mu-sofia.bg.
Comparison of treatment of COVID-19 with inhaled bromhexine, higher doses of colchicine and hymecromone with WHO-recommended paxlovid, molnupiravir, remdesivir, anti-IL-6 receptor antibodies and baricitinib
Pharmacia, doi:10.3897/pharmacia.70.e112550
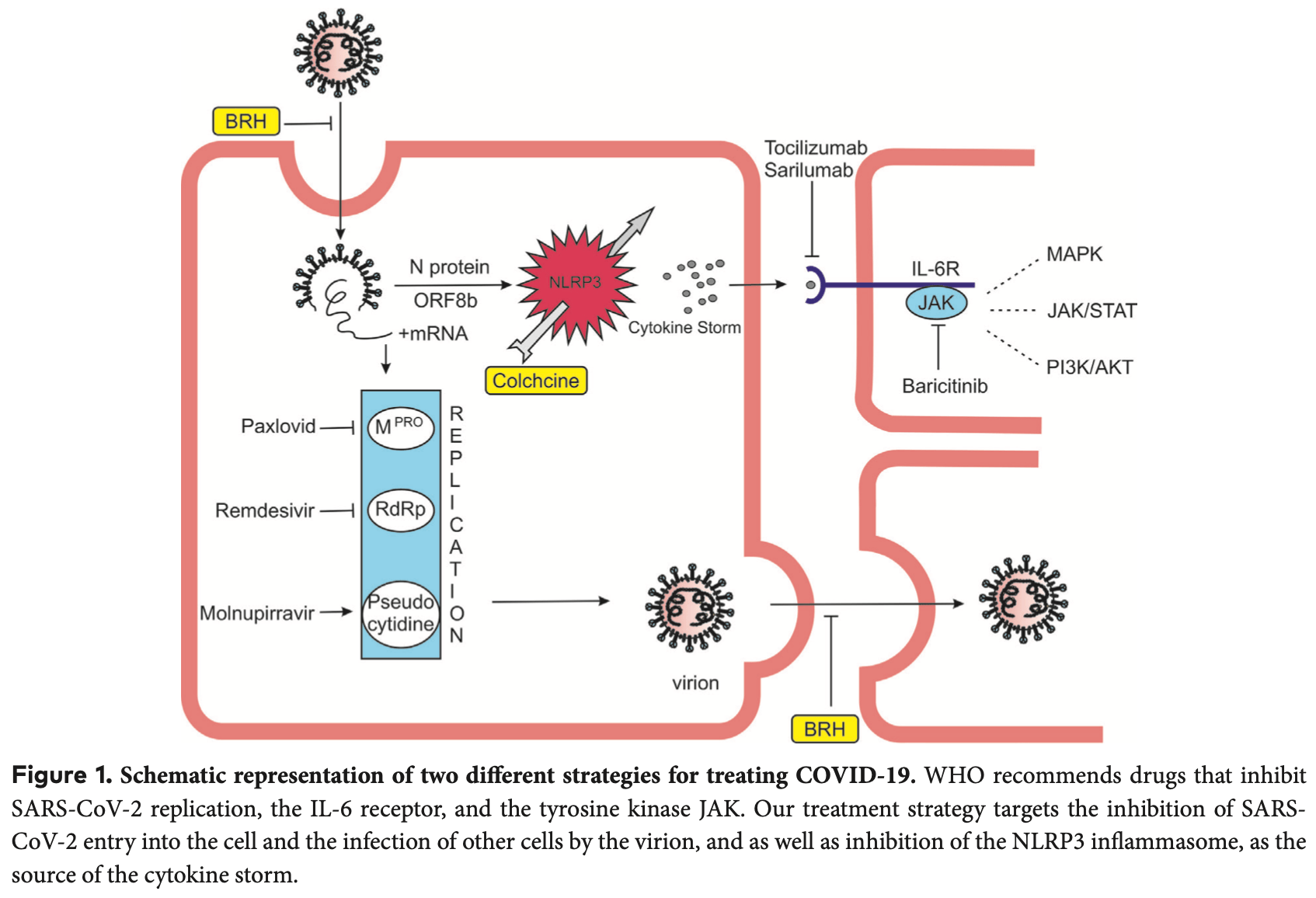
Millions of publications and thousands of clinical trials have not led to the discovery of an effective treatment for COVID-19. We believe that the reason for this is the inaccurate strategy of inhibiting target molecules involved in the pathogenesis of the disease. The leading cause of death in COVID-19 is the cytokine storm, which is caused by an NLRP3 inflammasome hyperreaction. WHO recommends for the outpatients treatment drugs blocking the replication of SARS-CoV-2. However, viral load and replication are not directly related to NLRP3 inflammasome hyperreactivity. This also explains the partial success of the WHO favorite paxlovid to reduce hospitalizations (51%). For hospital treatment, WHO suggests antibodies against the interleukin-6 receptor and Janus kinase (JAK) inhibition. Although important, IL-6 is one of dozens of cytokines elevated as a consequence of cytokine storm. The JAK inhibitor baricitinib inhibited the effect of not only IL-6 but also other elevated cytokines. But if the NLRP3 inflammasome is inhibited, the cytokines will not be elevated, and there will be no need for baricitinib. All medicines recommended by the WHO are distinguished by their very high prices. Our therapeutic strategy is based on inhibition of SARS-CoV-2 entry into the cell and inhibition of the NLRP3 inflammasome. We offer two readily available, cheap and well-known medications -bromhexine hydrochloride and colchicine. The many studies on the treatment of COVID-19 so far have not produced the expected result. The devil is buried in the details. For bromhexine, the reason is the way and its late application. Bromhexine is most effective when given prophylactically or started by inhalation after contact with a person with COVID-19. Its earliest possible application is crucial for its effect. Increased doses of colchicine are necessary for COVID-19 treatment due to the fact that it accumulates in leukocytes, and this leads to inhibition of NLRP3. The high doses we administer have been given widely in the past and are completely safe. Our highest dose is about 5 times lower per kg of weight than the lowest severe toxic dose of colchicine described. Our results show about a 5-fold decrease in hospital mortality and almost complete prevention of hospitalizations if outpatients are treated with inhaled bromhexine and colchicine.
References
Aghabiklooei, Zamani, Hassanian-Moghaddam, Nasouhi, Mashayekhian, Acute colchicine overdose: report of three cases, Reumatismo, doi:10.4081/reumatismo.2013.720
Ahern, Reid, Gordon, Mccredie, Brooks et al., Does colchicine work? The results of the first controlled study in acute gout, Australian and New Zealand Journal of Medicine, doi:10.1111/j.1445-5994.1987.tb01232.x
Ahmed-Khan, Matar, Coombes, Moin, Joseph et al., Remdesivir-associated acute liver failure in a covid-19 patient: a case report and literature review, Cureus, doi:10.7759/cureus.34221
Andersen, Rambaut, Lipkin, Holmes, Garry, The proximal origin of SARS-CoV-2, Nature Medicine, doi:10.1038/s41591-020-0820-9
Ansarin, Tolouian, Ardalan, Taghizadieh, Varshochi et al., Effect of bromhexine on clinical outcomes and mortality in COVID-19 patients: A randomized clinical trial, Bioimpacts, doi:10.34172/bi.2020.27
Bahadoram, Keikhaei, Bahadoram, Mahmoudian-Sani, Hassanzadeh et al., Bromhexine is a potential drug for COVID-19; From hypothesis to clinical trials, Vopr Virusol, doi:10.36233/0507-4088-106
Barry, The site of origin of the 1918 influenza pandemic and its public health implications, Journal of Translational Medicine, doi:10.1186/1479-5876-2-3
Barzegar, Ghadipasha, Rezaei, Forouzesh, Valizadeh, New hope for treatment of respiratory involvement following COVID-19 by bromhexine, Journal of Nephropharmacology, doi:10.34172/npj.2021.11
Baud, Sabouraud, Vicaut, Taboulet, Lang et al., Brief report: treatment of severe colchicine overdose with colchicine-specific Fab fragments, The New England Journal of Medicine, doi:10.1056/NEJM199503093321004
Broz, Dixit, Inflammasomes: mechanism of assembly, regulation and signalling, Nature Reviews Immunology, doi:10.1038/nri.2016.58
Centola, Wood, Frucht, Galon, Aringer et al., The gene for familial Mediterranean fever, MEFV, is expressed in early leukocyte development and is regulated in response to inflammatory mediators, Blood, The Journal of the American Society of Hematology, doi:10.1182/blood.V95.10.3223.010k26_3223_3231
Chappey, Niel, Dervichian, Wautier, Scherrmann et al., Colchicine concentration in leukocytes of patients with familial Mediterranean fever, British Journal of Clinical Pharmacology, doi:10.1111/j.1365-2125.1994.tb04328.x
Chegini, Bolurian, Mojtahed, Hafizi, High risk individuals in COVID-19 pandemic; an updated review, Immunopathol Per, doi:10.34172/ipp.2021.25
Chen, Wu, Guo, Cao, Huang et al., Clinical and immunologic features in severe and moderate Coronavirus Disease, Journal of Clinical Investigation, doi:10.1172/JCI137244
Chia, Grainger, Harper, Colchicine suppresses neutrophil superoxide production in a murine model of gouty arthritis: a rationale for use of low-dose colchicine, British Journal of Pharmacology, doi:10.1038/bjp.2008.20
Courjon, Dufies, Robert, Bailly, Torre et al., Heterogeneous nlrp3 inflammasome signature in circulating myeloid cells as a biomarker of COVID-19 severity, Blood Advances, doi:10.1182/bloodadvances.2020003918
Cronstein, Molad, Reibman, Balakhane, Levin et al., Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils, The Journal of Clinical Investigation, doi:10.1172/JCI118147
Cronstein, Sunkureddi, Practical reports on rheumatic & musculoskeletal diseases: Mechanistic aspects of inflammation and clinical management of inflammation in acute gouty arthritis, Journal of Clinical Rheumatology, doi:10.1097/RHU.0b013e31827d8790
Delorey, Ziegler, Heimberg, Yang, Segerstolpe et al., COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets, Nature, doi:10.1038/s41586-021-03570-8
Depfenhart, De Villiers, Lemperle, Meyer, Somma, Potential new treatment strategies for COVID-19: Is there a role for bromhexine as add-on therapy?, Internal and Emergency Medicine, doi:10.1007/s11739-020-02383-3
Ding, Porteu, Sanchez, Nathan, Downregulation of tumor necrosis factor receptors on macrophages and endothelial cells by microtubule depolymerizing agents, The Journal of experimental Medicine, doi:10.1084/jem.171.3.715
Dupuis, Sirois, Rhéaume, Nguyen, Clavet-Lanthier et al., Colchicine reduces lung injury in experimental acute respiratory distress syndrome, PLoS ONE, doi:10.1371/journal.pone.0242318
Ferreira, Soares, De Azevedo-Quintanilha, Dias, Fintelman-Rodrigues et al., SARS-CoV-2 engages inflammasome and pyroptosis in human primary monocytes, Cell Death Discovery, doi:10.1038/s41420-021-00428-w
Finkelstein, Aks, Hutson, Juurlink, Nguyen et al., Colchicine poisoning: the dark side of an ancient drug, Clinical Toxicology, doi:10.3109/15563650.2010.495348
Freeman, Swartz, Targeting the NLRP3 inflammasome in severe COVID-19, Frontiers in Immunology, doi:10.3389/fimmu.2020.01518
Fu, Wang, Yuan, Chen, Ao et al., Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis, Journal of Infection, doi:10.1016/j.jinf.2020.03.041
Fu, Xu, Wei, Why tocilizumab could be an effective treatment for severe COVID-19, Journal of Translational Medicine, doi:10.1186/s12967-020-02339-3
Fu, Zheng, Zhou, Tang, Chen et al., Re-recognizing bromhexine hydrochloride: pharmaceutical properties and its possible role in treating pediatric COVID-19, European Journal of Clinical Pharmacology, doi:10.1007/s00228-020-02971-4
Fung, Yuen, Ye, Chan, Jin, A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses, Emerging Microbes & Infections, doi:10.1080/22221751.2020.1736644
Gagnon, Miller, Hallman, Bourbeau, Herring et al., Age-specific mortality during the 1918 influenza pandemic: unravelling the mystery of high young adult mortality, PLoS ONE, doi:10.1371/journal.pone.0069586
Ganter, Roux, Miyazawa, Howard, Frank et al., Interleukin-1beta causes acute lung injury via alphavbeta5 and alphavbeta6 integrin-dependent mechanisms, Circulation Research, doi:10.1161/CIRCRESAHA.107.161067
Gholaminejhad, Forouzesh, Ebrahimi, Mahdavi, Mirtorabi et al., Formation and activity of NLRP3 inflammasome and histopathological changes in the lung of corpses with COVID-19, Journal of Molecular Histology, doi:10.1007/s10735-022-10101-w
Gottlieb, Vaca, Paredes, Mera, Webb et al., Early remdesivir to prevent progression to severe Covid-19 in outpatients, New England Journal of Medicine, doi:10.1056/NEJMoa2116846
Grasselli, Zangrillo, Zanella, Antonelli, Cabrini et al., Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy, JAMA, doi:10.1001/jama.2020.5394
Hansten, Tan, Horn, Gomez-Lumbreras, Villa-Zapata et al., Colchicine drug interaction errors and misunderstandings: Recommendations for improved evidence-based management, Drug Safety, doi:10.1007/s40264-022-01265-1
Hasanvand, COVID-19 and the role of cytokines in this disease, Inflammopharmacology, doi:10.1007/s10787-022-00992-2
He, Lau, Wu, Deng, Wang et al., Temporal dynamics in viral shedding and transmissibility of COVID-19, Nature Medicine, doi:10.1038/s41591-020-0869-5
Herold, Mayer, Lohmeyer, Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair, Frontiers in Immunology, doi:10.3389/fimmu.2011.00065
Hirano, Murakami, COVID-19: a new virus, but a familiar receptor and cytokine release syndrome, Immunity, doi:10.1016/j.immuni.2020.04.003
Hirayama, Hiruma, Ueda, Doi, Morimura, A critically ill patient after a colchicine overdose below the lethal dose: a case report, Journal of Medical Case Reports, doi:10.1186/s13256-018-1737-5
Hm, Niemi, Hussain, Gareeb, Lugnier, The potential role of Bromhexine in the management of COVID-19: Decipher and a real gamechanger, Current Medical and Drug Research, doi:10.53517/CMDR.2581-5008.512021212
Hoffmann, Kleine-Weber, Pöhlmann, A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells, Molecular Cell, doi:10.1016/j.molcel.2020.04.022
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Hojyo, Uchida, Tanaka, Hasebe, Tanaka et al., How COVID-19 induces cytokine storm with high mortality, Inflammation and Regeneration, doi:10.1186/s41232-020-00146-3
Hou, Okuda, Edwards, Martinez, Asakura et al., SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract, Cell, doi:10.1016/j.cell.2020.05.042
Hsu, Yu, Peng, Ye, Hu et al., The role of cytokines and chemokines in severe acute respiratory syndrome Coronavirus 2 infections, Frontiers in Immunology, doi:10.3389/fimmu.2022.832394
Hu, Pan, Li, He, Zhang et al., Increased circulating cytokines have a role in COVID-19 severity and death with a more pronounced effect in males: a systematic review and meta-analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.802228
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, The Lancet, doi:10.1016/S0140-6736(20)30183-5
Jackman, Rhoads, Cornwell, Kandarian, Microtubule-mediated NF-κB activation in the TNF-α signaling pathway, Experimental Cell Research, doi:10.1016/j.yexcr.2009.08.020
Jamilloux, Henry, Belot, Viel, Fauter et al., Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions, Autoimmunity Reviews, doi:10.1016/j.autrev.2020.102567
Jarvie, Park, Stewart, Estimation of colchicine in a poisoned patient by using high performance liquid chromatography, Clinical Toxicology, doi:10.3109/15563657909010599
Junqueira, Crespo, Ranjbar, De Lacerda, Lewandrowski et al., FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation, Nature, doi:10.1038/s41586-022-04702-4
Kaivola, Nyman, Matikainen, Inflammasomes and SARS-CoV-2 Infection, Viruses, doi:10.3390/v13122513
Kelley, Jeltema, Duan, He, The NLRP3 inflammasome: an overview of mechanisms of activation and regulation, International Journal Of Molecular Sciences, doi:10.3390/ijms20133328
Keyla Sg De Sá, Amaral, Caetano, Becerra, Batah et al., Inflammasome activation and pulmonary viral loads define two distinct clinical outcomes in COVID-19, MedRxiv, doi:10.1101/2022.06.24.22276878
Kleer, Willems, Lambrecht, Goriely, Ontogeny of myeloid cells, Frontiers in Immunology, doi:10.3389/fimmu.2014.00423
Knoll, Schultze, Schulte-Schrepping, Monocytes and Macrophages in COVID-19, Frontiers in Immunology, doi:10.3389/fimmu.2021.720109
Kolb, Margetts, Anthony, Pitossi, Gauldie, Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis, Journal of Clinical Investigation, doi:10.1172/JCI12568
Kovalchuk, Wang, Li, Rodriguez-Juarez, Ilnytskyy et al., Fighting the storm: could novel anti-TNFα and anti-IL-6 C. sativa cultivars tame cytokine storm in COVID-19?, Aging, doi:10.18632/aging.202500
Kuijpers, Raleigh, Kavanagh, Janssen, Calafat et al., Cytokine-activated endothelial cells internalize E-selectin into a lysosomal compartment of vesiculotubular shape. A tubulin-driven process, Journal of immunology, doi:10.4049/jimmunol.152.10.5060
Kumar, Nicholls, Wong, Partners in crime: neutrophils and monocytes/macrophages in inflammation and disease, Cell and Tissue Research, doi:10.1007/s00441-017-2753-2
Leung, Hui, Kraus, Colchicine: Update on mechanisms of action and therapeutic uses, Seminars in Arthritis and Rheumatism, doi:10.1016/j.semarthrit.2015.06.013
Li, Receptor recognition mechanisms of coronaviruses: a decade of structural studies, Journal of Virology, doi:10.1128/JVI.02615-14
Liu, Zhang, Huang, Yang, Wang et al., Elevated plasma levels of selective cytokines in COVID-19 patients reflect viral load and lung injury, National Science Review, doi:10.1093/nsr/nwaa037
Lu, Zhao, Li, Niu, Yang et al., Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding, The Lancet, doi:10.1016/S0140-6736(20)30251-8
Lucas, Heinlein, Kim, Hernandez, Malik et al., The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microen-vironment and promotes prostate cancer metastasis, Cancer Discovery, doi:10.1158/2159-8290.CD-13-1010
Macleod, Phillips, Hypersensitivity to colchicin, Annals of the Rheumatic Diseases, doi:10.1136/ard.6.4.224
Maes, Jr, Lozovoy, Mori, Danelli et al., In COVID-19, NLRP3 inflammasome genetic variants are associated with critical disease and these effects are partly mediated by the sickness symptom complex: a nomothetic network approach, Molecular Psychiatry, doi:10.1038/s41380-021-01431-4
Mahase, Covid-19: Pfizer's paxlovid is 89% effective in patients at risk of serious illness, company reports, British Medical Journal, doi:10.1136/bmj.n2713
Martinon, Pétrilli, Mayor, Tardivel, Tschopp, Gout-associated uric acid crystals activate the NALP3 inflammasome, Nature, doi:10.1038/nature04516
Martínez, Robertson, Barraclough, Xia, Mallat et al., Colchicine acutely auppresses local cardiac production of inflammatory cytokines in patients with an acute coronary syndrome, Journal of the American Heart Association, doi:10.1161/JAHA.115.002128
Matheson, Lehner, How does SARS-CoV-2 cause COVID-19?, Science, doi:10.1126/science.abc6156
Mcgonagle, Sharif, 'regan, Bridgewood, The role of cytokines including Interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease, Autoimmunity Reviews, doi:10.1016/j.autrev.2020.102537
Mehta, Mcauley, Brown, Sanchez, Tattersall et al., COVID-19: consider cytokine storm syndromes and immunosuppression, The Lancet, doi:10.1016/S0140-6736(20)30628-0
Melms, Biermann, Huang, Wang, Nair et al., A molecular single-cell lung atlas of lethal COVID-19, Nature, doi:10.1038/s41586-021-03569-1
Merad, Martin, Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages, Nature Reviews Immunology, doi:10.1038/s41577-020-0331-4
Mikhaylov, Lyubimtseva, Vakhrushev, Stepanov, Lebedev et al., Bromhexine hydrochloride prophylaxis of COVID-19 for medical personnel: A randomized open-label study, Interdisciplinary Perspectives on Infectious Diseases, doi:10.1155/2022/4693121
Mikolajewska, Fischer, Piechotta, Mueller, Metzendorf et al., Colchicine for the treatment of COVID-19, Cochrane Database of Systematic Reviews, doi:10.1002/14651858.CD015045
Misawa, Takahama, Kozaki, Lee, Zou et al., Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome, Nature Immunology, doi:10.1038/ni.2550
Mitev, Mondeshki, Bilukov, Colchicine optimal doses for COVID-19 treatment are not life-threatening, Medizina i Sport
Mitev, Mondeshki, Marinov, Bilukov, Colchicine, bromhexine, and hymecromone as part of COVID19 treatmentcold, warm, hot, doi:10.9734/bpi/codhr/v10/5310A
Mitev, Mondeshki, Marinov, Inhaled BRH, optimal doses of colchicine and hymecromone are three powerful weapons for prevention, outpation and inpation treatment of Covid-19, Medizina i Sport
Mitev, Mondeshki, Treatment of COVID-19 with colchicine and inhaled bromhexine, Medizina i Sport
Mitev, Outpatient treatment of Covid-19, Medizina i Sport
Momekov, Momekova, Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view: antiviral levels are not likely attainable with known dosing regimens, Biotechnology & Biotechnological Equipment, doi:10.1080/13102818.2020.1775118
Mondeshki, Bilyukov, Mitev, Effect of an accidental colchicine overdose in a COVID-19 inpatient with bilateral pneumonia and pericardial effusion, Cureus, doi:10.7759/cureus.35909
Mondeshki, Bilyukov, Tomov, Mihaylov, Mitev, Complete, rapid resolution of severe bilateral pneumonia and acute respiratory distress syndrome in a COVID-19 patient: role for a unique therapeutic combination of inhalations with bromhexine, higher doses of colchicine, and hymecromone, Cureus, doi:10.7759/cureus.30269
Nerlekar, Beale, Harper, Colchicine -a short history of an ancient drug, The Medical journal of Australia, doi:10.5694/mja14.00846
Oka, Hori, Ozaki, Microtubule disruption suppresses allergic response through the inhibition of calcium influx in the mast cell degranulation pathway, The Journal of Immunology, doi:10.4049/jimmunol.174.8.4584
Ontong, Prachayasittikul, Unraveled roles of hyaluronan in severe COVID-19, EXCLI Journal
Otani, Watanabe, Shimada, Takeda, Itani et al., Colchicine prevents NSAID-induced small intestinal injury by inhibiting activation of the NLRP3 inflammasome, Scientific Reports, doi:10.1038/srep32587
Ozen, Demirkaya, Erer, Livneh, Ben-Chetrit et al., Eular recommendations for the management of familial Mediterranean fever, Annals of the Rheumatic Diseases, doi:10.1136/annrheumdis-2015-208690
Pan, Shen, Yu, Ge, Chen et al., SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation, Nature Communications, doi:10.1038/s41467-021-25015-6
Pascart, Lancrenon, Lanz, Delva, Guggenbuhl et al., GOSPEL 2 -Colchicine for the treatment of gout flares in France -a GOSPEL survey subgroup analysis. Doses used in common practices regardless of renal impairment and age, Joint Bone Spine, doi:10.1016/j.jbspin.2015.10.006
Paschke, Weidner, Paust, Marti, Beil et al., Technical advance: inhibition of neutrophil chemotaxis by colchicine is modulated through viscoelastic properties of subcellular compartments, Journal of Leukocyte Biology, doi:10.1189/jlb.1012510
Patton, Saggart, Ahmed, Leff, Repine, Interleukin-1β-induced neutrophil recruitment and acute lung injury in hamsters, Inflammation, doi:10.1007/BF01534377
Perricone, Scarsi, Brucato, Pisano, Pigatto et al., COLVID-19 study group, under the auspices of the Italian Society of Rheumatology (SIR), the Italian Society of Infectious and Tropical Diseases (SIMIT) and the Italian Thoracic Society (ITS-AIPO) (2023) Treatment with COLchicine in hospitalized patients affected by COVID-19: The COLVID-19 trial, European Journal of Internal Medicine, doi:10.1016/j.ejim.2022.10.016
Pontelli, Castro, Martins, Serra, Veras et al., SARS-CoV-2 productively infects primary human immune system cells in vitro and in COVID-19 patients, Molecular and Cellular Biology, doi:10.1093/jmcb/mjac021
Pérez, Prod'hom, De Villiers, Ferry, Amiet et al., Case report: Colchicine toxicokinetic analysis in a poisoned child requiring extracorporeal life support, Frontiers in Pediatrics, doi:10.3389/fped.2021.658347
Roberge, Gaudry, Gilbert, Malawista, Medicis et al., Paradoxical effects of colchicine on the activation of human neutrophils by chemotactic factors and inflammatory microcrystals, Journal of Leukocyte Biology, doi:10.1002/jlb.59.6.864
Robertson, Martínez, Payet, Barraclough, Celermajer et al., Colchicine therapy in acute coronary syndrome patients acts on caspase-1 to suppress NLRP3 inflammasome monocyte activation, Clinical Science, doi:10.1042/CS20160090
Rodrigues, De Sá, Ishimoto, Becerra, Oliveira et al., Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients, Journal of Experimental Medicine, doi:10.1084/jem.20201707
Ruan, Yang, Wang, Jiang, Song, Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China, Intensive Care Medicine, doi:10.1007/s00134-020-05991-x
Rudolph, Greengard, Malawista, Effects of colchicine on cyclic AMP levels in human leukocytes, Proceedings of the National Academy of Sciences, doi:10.1073/pnas.74.8.3404
Safi, Kallas, Bardawil, Mehanna, Abbas et al., Neutrophils contribute to vasculitis by increased release of neutrophil extracellular traps in Behcet's disease, Journal of Dermatological Science, doi:10.1016/j.jdermsci.2018.08.010
Sayarlioglu, Dogan, Erkoc, Ozbek, Bayram et al., The effect of colchicine on the peritoneal membrane, Renal Failure, doi:10.1080/08860220500461286
Scarsi, Piantoni, Colombo, Airó, Richini et al., Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome, Annals of the Rheumatic Diseases, doi:10.1136/annrheumdis-2020-217712
Schulert, Grom, Pathogenesis of macrophage activation syndrome and potential for cytokine-directed therapies, Annual Review of Medicine, doi:10.1146/annurev-med-061813-012806
Sefik, Qu, Junqueira, Kaffe, Mirza et al., Inflammasome activation in infected macrophages drives COVID-19 pathology, Nature, doi:10.1038/s41586-022-04802-1
Shah, Joyce, Plumb, Sahakian, Feldstein et al., Paxlovid associated with decreased hospitalization rate among adults with COVID-19 -United States, April-September, American Journal of Transplantation, doi:10.1016/j.ajt.2022.12.004
Sherline, Leung, Kipnis, Binding of colchicine to purified microtubule protein, Journal of Biological Chemistry, doi:10.1016/S0021-9258(19)41207-6
Shimizu, Clinical features of cytokine storm syndrome In: Cron RQ, doi:10.1007/978-3-030-22094-5_3
Siddiqi, Mehra, COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal, The Journal of Heart and Lung Transplantation, doi:10.1016/j.healun.2020.03.012
Silva, Wanderley, Veras, Gonçalves, Lima et al., Gasdermin-D activation by SARS-CoV-2 Triggers NET and Mediate COVID-19 Immunopathology, Critical Care, doi:10.1186/s13054-022-04062-5
Stack, Ryan, Mccarthy, Colchicine: New Insights to an Old Drug, American Journal of Therapeutics, doi:10.1097/01.mjt.0000433937.07244.e1
Sun, Liu, Huang, Xu, Hu et al., SARS-CoV-2 non-structural protein 6 triggers NLRP3-dependent pyroptosis by targeting ATP6AP1, Cell Death & Differentiation, doi:10.1038/s41418-021-00916-7
Tardif, Bouabdallaoui, Allier, Gaudet, Shah et al., Colchicine for community-treated patients with Covid-19 (Colcorona): A phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial, The Lancet Respiratory Medicine, doi:10.1016/S2213-2600(21)00222-8
Tardif, Kouz, Waters, Bertrand, Diaz et al., Efficacy and safety of low-dose colchicine after myocardial infarction, The New England Journal of Medicine, doi:10.1056/NEJMoa1912388
Tartof, Qian, Hong, Wei, Nadjafi et al., Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization, Annals of Internal Medicine, doi:10.7326/M20-3742
Taylor, The mechanism of colchicine inhibition of mitosis: I. Kinetics of inhibition and the binding of h3-colchicine, The Journal of Cell Biology, doi:10.1083/jcb.25.1.145
Terkeltaub, Colchicine update: 2008, Seminars in Arthritis and Rheumatism, doi:10.1016/j.semarthrit.2008.08.006
Terkeltaub, Furst, Bennett, Kook, Crockett et al., High versus low dosing of oral colchicine for early acute gout flare: Twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study, Arthritis & Rheumatology, doi:10.1002/art.27327
To, Tsang, Leung, Tam, Wu et al., Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(20)30196-1
Tolouian, Moradi, Mulla, Ziaie, Haghighi et al., Bromhexine for post-exposure COVID-19 prophylaxis: A randomized, double-blind, placebo-controlled trial, Jundishapur Journal of Microbiology, doi:10.21203/rs.3.rs-1612290/v1
Tolouian, Mulla, Controversy with bromhexine in COVID-19; where we stand, Immunopathologia Persa, doi:10.34172/ipp.2021.12
Tolouian, Mulla, Jamaati, Babamahmoodi, Marjani et al., Effect of bromhexine in hospitalized patients with COVID-19, Journal of Investigative Medicine, doi:10.1136/jim-2020-001747
Vaidya, Tucker, Kurup, Khandkar, Pandzic et al., Colchicine inhibits neutrophil extracellular trap formation in patients with acute coronary syndrome after percutaneous coronary intervention, Journal of the American Heart Association, doi:10.1161/JAHA.120.018993
Vila Méndez, Sanz, García, Malo, Martínez et al., Efficacy of bromhexine versus standard of care in reducing viral load in patients with mild-to-moderate COVID-19 disease attended in primary care: A randomized open-label trial, Journal of Clinical Medicine, doi:10.3390/jcm12010142
Vitiello, Ferrara, Colchicine and SARS-CoV-2: Management of the hyperinflammatory state, Respiratory Medicine, doi:10.1016/j.rmed.2021.106322
Wallace, Ertel, Occupancy approach to colchicine dosage, The Lancet, doi:10.1016/S0140-6736(70)92206-3
Wang, Hu, Hu, Zhu, Liu et al., Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China, JAMA, doi:10.1001/jama.2020.1585
Wettstein, Kirchhoff, Münch, Remdesivir and three other drugs for hospitalized patients with COVID-19: final results of the WHO Solidarity randomized trial and updated meta-analyses, International Journal of Molecular Sciences, doi:10.1016/S0140-6736(22)00519-0
Who Solidarity, Consortium, Pan, Peto, Henao-Restrepo et al., Repurposed antiviral drugs for covid-19 -interim who solidarity trial results, The New England Journal of Medicine, doi:10.1056/NEJMoa2023184
Wong, Leong, Lee, Albani, Yeo, Insights into the immuno-pathogenesis of acute respiratory distress syndrome, Annals of Translational Medicine, doi:10.21037/atm.2019.09.28
Wrapp, Wang, Corbett, Goldsmith, Hsieh et al., Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation, Science, doi:10.1126/science.abb2507
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention, Jama, doi:10.1001/jama.2020.2648
Wölfel, Corman, Guggemos, Seilmaier, Zange et al., Virological assessment of hospitalized patients with COVID-2019, Nature, doi:10.1038/s41586-020-2196-x
Xu, Akinyemi, Chitre, Loeb, Lednicky et al., SARS-CoV-2 viroporin encoded by ORF3a triggers the NLRP3 inflammatory pathway, Virology, doi:10.1016/j.virol.2022.01.003
Xu, Shi, Wang, Zhang, Huang et al., Pathological findings of Covid-19 associated with acute respiratory distress syndrome, The Lancet Respiratory Medicine, doi:10.1016/S2213-2600(20)30076-X
Yagnik, Evans, Florey, Mason, Landis et al., Official Journal of the American College of Rheumatology: Macrophage release of transforming growth factor β1 during resolution of monosodium urate monohydrate crystal-induced inflammation, Arthritis & Rheumatism, doi:10.1002/art.20317
Yahia, Ben Zvi, Livneh, Colchicine intoxication in familial Mediterranean fever patients using clarithromycin for the treatment of Helicobacter pylori: a series of six patients, Rheumatology International, doi:10.1007/s00296-017-3823-1
Yang, Tong, Chen, Yu, Human Identical Sequences, hyaluronan, and hymecromone -The new mechanism and management of COVID-19, Molecular Biomedicine, doi:10.1186/s43556-022-00077-0
Zhang, Dong, Cao, Yuan, Yang et al., Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China, Allergy, doi:10.1111/all.14238
Zhang, Wu, Li, Zhao, Wang, Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality, International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2020.105954
Zhao, Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies, International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2020.105982
Zhao, Di, Xu, The NLRP3 inflammasome and COVID-19: Activation, pathogenesis and therapeutic strategies, Cytokine & Growth Factor Reviews, doi:10.1016/j.cytogfr.2021.06.002
Zhou, Yang, Wang, Hu, Zhang et al., A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature, doi:10.1038/s41586-020-2012-7
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, The Lancet, doi:10.1016/S0140-6736(20)30566-3
DOI record:
{
"DOI": "10.3897/pharmacia.70.e112550",
"ISSN": [
"2603-557X",
"0428-0296"
],
"URL": "http://dx.doi.org/10.3897/pharmacia.70.e112550",
"abstract": "<jats:p>Millions of publications and thousands of clinical trials have not led to the discovery of an effective treatment for COVID-19. We believe that the reason for this is the inaccurate strategy of inhibiting target molecules involved in the pathogenesis of the disease.</jats:p>\n <jats:p>The leading cause of death in COVID-19 is the cytokine storm, which is caused by an NLRP3 inflammasome hyperreaction. WHO recommends for the outpatients treatment drugs blocking the replication of SARS-CoV-2. However, viral load and replication are not directly related to NLRP3 inflammasome hyperreactivity. This also explains the partial success of the WHO favorite paxlovid to reduce hospitalizations (51%). For hospital treatment, WHO suggests antibodies against the interleukin-6 receptor and Janus kinase (JAK) inhibition. Although important, IL-6 is one of dozens of cytokines elevated as a consequence of cytokine storm. The JAK inhibitor baricitinib inhibited the effect of not only IL-6 but also other elevated cytokines. But if the NLRP3 inflammasome is inhibited, the cytokines will not be elevated, and there will be no need for baricitinib. All medicines recommended by the WHO are distinguished by their very high prices.</jats:p>\n <jats:p>Our therapeutic strategy is based on inhibition of SARS-CoV-2 entry into the cell and inhibition of the NLRP3 inflammasome. We offer two readily available, cheap and well-known medications - bromhexine hydrochloride and colchicine. The many studies on the treatment of COVID-19 so far have not produced the expected result. The devil is buried in the details.</jats:p>\n <jats:p>For bromhexine, the reason is the way and its late application. Bromhexine is most effective when given prophylactically or started by inhalation after contact with a person with COVID-19. Its earliest possible application is crucial for its effect.</jats:p>\n <jats:p>Increased doses of colchicine are necessary for COVID-19 treatment due to the fact that it accumulates in leukocytes, and this leads to inhibition of NLRP3. The high doses we administer have been given widely in the past and are completely safe. Our highest dose is about 5 times lower per kg of weight than the lowest severe toxic dose of colchicine described. Our results show about a 5-fold decrease in hospital mortality and almost complete prevention of hospitalizations if outpatients are treated with inhaled bromhexine and colchicine.</jats:p>",
"author": [
{
"ORCID": "http://orcid.org/0000-0001-7528-590X",
"affiliation": [],
"authenticated-orcid": true,
"family": "Mitev",
"given": "Vanyo",
"sequence": "first"
}
],
"container-title": "Pharmacia",
"container-title-short": "PHAR",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
10,
20
]
],
"date-time": "2023-10-20T13:50:25Z",
"timestamp": 1697809825000
},
"deposited": {
"date-parts": [
[
2023,
10,
20
]
],
"date-time": "2023-10-20T13:50:34Z",
"timestamp": 1697809834000
},
"funder": [
{
"DOI": "10.13039/501100003336",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100003336",
"id-type": "DOI"
}
],
"name": "Bulgarian National Science Fund"
}
],
"indexed": {
"date-parts": [
[
2024,
8,
26
]
],
"date-time": "2024-08-26T17:05:56Z",
"timestamp": 1724691956755
},
"is-referenced-by-count": 9,
"issue": "4",
"issued": {
"date-parts": [
[
2023,
10,
20
]
]
},
"journal-issue": {
"issue": "4",
"published-online": {
"date-parts": [
[
2023,
10,
20
]
]
}
},
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
10,
20
]
],
"date-time": "2023-10-20T00:00:00Z",
"timestamp": 1697760000000
}
}
],
"link": [
{
"URL": "https://pharmacia.pensoft.net/article/112550/download/pdf/",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://pharmacia.pensoft.net/article/112550/download/xml/",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://pharmacia.pensoft.net/article_preview.php?id=112550&skip_redirect=1",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "2258",
"original-title": [],
"page": "1177-1193",
"prefix": "10.3897",
"published": {
"date-parts": [
[
2023,
10,
20
]
]
},
"published-online": {
"date-parts": [
[
2023,
10,
20
]
]
},
"publisher": "Pensoft Publishers",
"reference": [
{
"DOI": "10.4081/reumatismo.2013.720",
"doi-asserted-by": "publisher",
"key": "112550_B1"
},
{
"DOI": "10.1111/j.1445-5994.1987.tb01232.x",
"doi-asserted-by": "publisher",
"key": "112550_B2"
},
{
"DOI": "10.7759/cureus.34221",
"doi-asserted-by": "publisher",
"key": "112550_B3"
},
{
"DOI": "10.53517/CMDR.2581-5008.512021212",
"doi-asserted-by": "publisher",
"key": "112550_B4"
},
{
"DOI": "10.1038/s41591-020-0820-9",
"doi-asserted-by": "publisher",
"key": "112550_B5"
},
{
"DOI": "10.34172/bi.2020.27",
"doi-asserted-by": "publisher",
"key": "112550_B6"
},
{
"DOI": "10.36233/0507-4088-106",
"doi-asserted-by": "publisher",
"key": "112550_B7"
},
{
"DOI": "10.1186/1479-5876-2-3",
"doi-asserted-by": "publisher",
"key": "112550_B8"
},
{
"DOI": "10.34172/npj.2021.11",
"doi-asserted-by": "publisher",
"key": "112550_B9"
},
{
"DOI": "10.1056/NEJM199503093321004",
"doi-asserted-by": "publisher",
"key": "112550_B10"
},
{
"DOI": "10.1038/nri.2016.58",
"doi-asserted-by": "publisher",
"key": "112550_B11"
},
{
"DOI": "10.1182/blood.V95.10.3223.010k26_3223_3231",
"doi-asserted-by": "publisher",
"key": "112550_B12"
},
{
"DOI": "10.1111/j.1365-2125.1994.tb04328.x",
"doi-asserted-by": "publisher",
"key": "112550_B13"
},
{
"DOI": "10.34172/ipp.2021.25",
"doi-asserted-by": "publisher",
"key": "112550_B14"
},
{
"DOI": "10.1172/JCI137244",
"doi-asserted-by": "publisher",
"key": "112550_B15"
},
{
"DOI": "10.1038/bjp.2008.20",
"doi-asserted-by": "publisher",
"key": "112550_B16"
},
{
"DOI": "10.1182/bloodadvances.2020003918",
"doi-asserted-by": "publisher",
"key": "112550_B17"
},
{
"DOI": "10.1172/JCI118147",
"doi-asserted-by": "publisher",
"key": "112550_B18"
},
{
"DOI": "10.1097/RHU.0b013e31827d8790",
"doi-asserted-by": "publisher",
"key": "112550_B19"
},
{
"DOI": "10.3389/fimmu.2014.00423",
"doi-asserted-by": "publisher",
"key": "112550_B20"
},
{
"DOI": "10.1038/s41586-021-03570-8",
"doi-asserted-by": "publisher",
"key": "112550_B21"
},
{
"DOI": "10.1007/s11739-020-02383-3",
"doi-asserted-by": "publisher",
"key": "112550_B22"
},
{
"DOI": "10.1084/jem.171.3.715",
"doi-asserted-by": "publisher",
"key": "112550_B23"
},
{
"DOI": "10.1371/journal.pone.0242318",
"doi-asserted-by": "publisher",
"key": "112550_B24"
},
{
"DOI": "10.1038/s41420-021-00428-w",
"doi-asserted-by": "publisher",
"key": "112550_B25"
},
{
"DOI": "10.3109/15563650.2010.495348",
"doi-asserted-by": "publisher",
"key": "112550_B26"
},
{
"DOI": "10.3389/fimmu.2020.01518",
"doi-asserted-by": "publisher",
"key": "112550_B27"
},
{
"DOI": "10.1186/s12967-020-02339-3",
"doi-asserted-by": "publisher",
"key": "112550_B28"
},
{
"DOI": "10.1016/j.jinf.2020.03.041",
"doi-asserted-by": "publisher",
"key": "112550_B29"
},
{
"DOI": "10.1007/s00228-020-02971-4",
"doi-asserted-by": "publisher",
"key": "112550_B30"
},
{
"DOI": "10.1080/22221751.2020.1736644",
"doi-asserted-by": "publisher",
"key": "112550_B31"
},
{
"DOI": "10.1371/journal.pone.0069586",
"doi-asserted-by": "publisher",
"key": "112550_B32"
},
{
"DOI": "10.1161/CIRCRESAHA.107.161067",
"doi-asserted-by": "publisher",
"key": "112550_B33"
},
{
"DOI": "10.1007/s10735-022-10101-w",
"doi-asserted-by": "publisher",
"key": "112550_B34"
},
{
"DOI": "10.1056/NEJMoa2116846",
"doi-asserted-by": "publisher",
"key": "112550_B35"
},
{
"DOI": "10.1001/jama.2020.5394",
"doi-asserted-by": "publisher",
"key": "112550_B36"
},
{
"DOI": "10.1007/s40264-022-01265-1",
"doi-asserted-by": "publisher",
"key": "112550_B37"
},
{
"DOI": "10.1007/s10787-022-00992-2",
"doi-asserted-by": "publisher",
"key": "112550_B38"
},
{
"DOI": "10.1038/s41591-020-0869-5",
"doi-asserted-by": "publisher",
"key": "112550_B39"
},
{
"DOI": "10.3389/fimmu.2011.00065",
"doi-asserted-by": "publisher",
"key": "112550_B40"
},
{
"DOI": "10.1016/j.immuni.2020.04.003",
"doi-asserted-by": "publisher",
"key": "112550_B41"
},
{
"DOI": "10.1186/s13256-018-1737-5",
"doi-asserted-by": "publisher",
"key": "112550_B42"
},
{
"DOI": "10.1016/j.molcel.2020.04.022",
"doi-asserted-by": "publisher",
"key": "112550_B43"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"doi-asserted-by": "publisher",
"key": "112550_B44"
},
{
"DOI": "10.1186/s41232-020-00146-3",
"doi-asserted-by": "publisher",
"key": "112550_B45"
},
{
"DOI": "10.1016/j.cell.2020.05.042",
"doi-asserted-by": "publisher",
"key": "112550_B46"
},
{
"DOI": "10.3389/fimmu.2022.832394",
"doi-asserted-by": "publisher",
"key": "112550_B47"
},
{
"DOI": "10.3389/fphar.2022.802228",
"doi-asserted-by": "publisher",
"key": "112550_B48"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"doi-asserted-by": "publisher",
"key": "112550_B49"
},
{
"DOI": "10.1016/j.yexcr.2009.08.020",
"doi-asserted-by": "publisher",
"key": "112550_B50"
},
{
"DOI": "10.1016/j.autrev.2020.102567",
"doi-asserted-by": "publisher",
"key": "112550_B51"
},
{
"DOI": "10.3109/15563657909010599",
"doi-asserted-by": "publisher",
"key": "112550_B52"
},
{
"DOI": "10.1038/s41586-022-04702-4",
"doi-asserted-by": "publisher",
"key": "112550_B53"
},
{
"DOI": "10.3390/v13122513",
"doi-asserted-by": "publisher",
"key": "112550_B54"
},
{
"DOI": "10.3390/ijms20133328",
"doi-asserted-by": "publisher",
"key": "112550_B55"
},
{
"DOI": "10.1101/2022.06.24.22276878",
"doi-asserted-by": "publisher",
"key": "112550_B56"
},
{
"DOI": "10.3389/fimmu.2021.720109",
"doi-asserted-by": "publisher",
"key": "112550_B57"
},
{
"DOI": "10.1172/JCI12568",
"doi-asserted-by": "publisher",
"key": "112550_B58"
},
{
"DOI": "10.18632/aging.202500",
"doi-asserted-by": "publisher",
"key": "112550_B59"
},
{
"DOI": "10.4049/jimmunol.152.10.5060",
"doi-asserted-by": "publisher",
"key": "112550_B60"
},
{
"DOI": "10.1016/j.semarthrit.2015.06.013",
"doi-asserted-by": "publisher",
"key": "112550_B61"
},
{
"DOI": "10.1128/JVI.02615-14",
"doi-asserted-by": "publisher",
"key": "112550_B62"
},
{
"DOI": "10.1093/nsr/nwaa037",
"doi-asserted-by": "publisher",
"key": "112550_B63"
},
{
"DOI": "10.1016/S0140-6736(20)30251-8",
"doi-asserted-by": "publisher",
"key": "112550_B64"
},
{
"DOI": "10.1158/2159-8290.CD-13-1010",
"doi-asserted-by": "publisher",
"key": "112550_B65"
},
{
"DOI": "10.1136/ard.6.4.224",
"doi-asserted-by": "publisher",
"key": "112550_B66"
},
{
"DOI": "10.1038/s41380-021-01431-4",
"doi-asserted-by": "publisher",
"key": "112550_B67"
},
{
"DOI": "10.1136/bmj.n2713",
"doi-asserted-by": "publisher",
"key": "112550_B68"
},
{
"DOI": "10.1161/JAHA.115.002128",
"doi-asserted-by": "publisher",
"key": "112550_B69"
},
{
"DOI": "10.1038/nature04516",
"doi-asserted-by": "publisher",
"key": "112550_B70"
},
{
"DOI": "10.1126/science.abc6156",
"doi-asserted-by": "publisher",
"key": "112550_B71"
},
{
"DOI": "10.1016/j.autrev.2020.102537",
"doi-asserted-by": "publisher",
"key": "112550_B72"
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"doi-asserted-by": "publisher",
"key": "112550_B73"
},
{
"DOI": "10.1038/s41586-021-03569-1",
"doi-asserted-by": "publisher",
"key": "112550_B74"
},
{
"DOI": "10.1038/s41577-020-0331-4",
"doi-asserted-by": "publisher",
"key": "112550_B75"
},
{
"DOI": "10.1155/2022/4693121",
"doi-asserted-by": "publisher",
"key": "112550_B76"
},
{
"DOI": "10.1002/14651858.CD015045",
"doi-asserted-by": "publisher",
"key": "112550_B77"
},
{
"DOI": "10.1038/ni.2550",
"doi-asserted-by": "publisher",
"key": "112550_B78"
},
{
"author": "Mitev",
"key": "112550_B79",
"year": "2022"
},
{
"author": "Mitev",
"key": "112550_B80",
"year": "2022"
},
{
"author": "Mitev",
"key": "112550_B81",
"year": "2023a"
},
{
"article-title": "Inhaled BRH, optimal doses of colchicine and hymecromone are three powerful weapons for prevention, outpation and inpation treatment of Covid-19.",
"author": "Mitev",
"first-page": "42",
"journal-title": "Medizina i Sport",
"key": "112550_B82",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.9734/bpi/codhr/v10/5310A",
"doi-asserted-by": "publisher",
"key": "112550_B83"
},
{
"DOI": "10.1080/13102818.2020.1775118",
"doi-asserted-by": "publisher",
"key": "112550_B84"
},
{
"DOI": "10.7759/cureus.35909",
"doi-asserted-by": "publisher",
"key": "112550_B85"
},
{
"DOI": "10.7759/cureus.30269",
"doi-asserted-by": "publisher",
"key": "112550_B86"
},
{
"DOI": "10.5694/mja14.00846",
"doi-asserted-by": "publisher",
"key": "112550_B87"
},
{
"DOI": "10.4049/jimmunol.174.8.4584",
"doi-asserted-by": "publisher",
"key": "112550_B88"
},
{
"article-title": "Unraveled roles of hyaluronan in severe COVID-19.",
"author": "Ontong",
"first-page": "117",
"journal-title": "EXCLI Journal",
"key": "112550_B89",
"volume": "20",
"year": "2021"
},
{
"DOI": "10.1038/srep32587",
"doi-asserted-by": "publisher",
"key": "112550_B90"
},
{
"DOI": "10.1136/annrheumdis-2015-208690",
"doi-asserted-by": "publisher",
"key": "112550_B91"
},
{
"DOI": "10.1038/s41467-021-25015-6",
"doi-asserted-by": "publisher",
"key": "112550_B92"
},
{
"DOI": "10.1016/j.jbspin.2015.10.006",
"doi-asserted-by": "publisher",
"key": "112550_B93"
},
{
"DOI": "10.1189/jlb.1012510",
"doi-asserted-by": "publisher",
"key": "112550_B94"
},
{
"DOI": "10.1007/BF01534377",
"doi-asserted-by": "publisher",
"key": "112550_B95"
},
{
"DOI": "10.3389/fped.2021.658347",
"doi-asserted-by": "publisher",
"key": "112550_B96"
},
{
"DOI": "10.1016/j.ejim.2022.10.016",
"doi-asserted-by": "publisher",
"key": "112550_B97"
},
{
"DOI": "10.1093/jmcb/mjac021",
"doi-asserted-by": "publisher",
"key": "112550_B98"
},
{
"DOI": "10.1007/s00441-017-2753-2",
"doi-asserted-by": "publisher",
"key": "112550_B99"
},
{
"DOI": "10.1016/S2213-2600(21)00435-5",
"doi-asserted-by": "publisher",
"key": "112550_B100"
},
{
"DOI": "10.1002/jlb.59.6.864",
"doi-asserted-by": "publisher",
"key": "112550_B101"
},
{
"DOI": "10.1042/CS20160090",
"doi-asserted-by": "publisher",
"key": "112550_B102"
},
{
"DOI": "10.1084/jem.20201707",
"doi-asserted-by": "publisher",
"key": "112550_B103"
},
{
"DOI": "10.1007/s00134-020-05991-x",
"doi-asserted-by": "publisher",
"key": "112550_B104"
},
{
"DOI": "10.1073/pnas.74.8.3404",
"doi-asserted-by": "publisher",
"key": "112550_B105"
},
{
"DOI": "10.1016/j.jdermsci.2018.08.010",
"doi-asserted-by": "publisher",
"key": "112550_B106"
},
{
"DOI": "10.1080/08860220500461286",
"doi-asserted-by": "publisher",
"key": "112550_B107"
},
{
"DOI": "10.1136/annrheumdis-2020-217712",
"doi-asserted-by": "publisher",
"key": "112550_B108"
},
{
"DOI": "10.1146/annurev-med-061813-012806",
"doi-asserted-by": "publisher",
"key": "112550_B109"
},
{
"DOI": "10.1038/s41586-022-04802-1",
"doi-asserted-by": "publisher",
"key": "112550_B110"
},
{
"DOI": "10.1016/j.ajt.2022.12.004",
"doi-asserted-by": "publisher",
"key": "112550_B111"
},
{
"DOI": "10.1016/S0021-9258(19)41207-6",
"doi-asserted-by": "publisher",
"key": "112550_B112"
},
{
"DOI": "10.1007/978-3-030-22094-5_3",
"doi-asserted-by": "publisher",
"key": "112550_B113"
},
{
"DOI": "10.1016/j.healun.2020.03.012",
"doi-asserted-by": "publisher",
"key": "112550_B114"
},
{
"DOI": "10.1186/s13054-022-04062-5",
"doi-asserted-by": "publisher",
"key": "112550_B115"
},
{
"DOI": "10.1097/01.mjt.0000433937.07244.e1",
"doi-asserted-by": "publisher",
"key": "112550_B116"
},
{
"DOI": "10.1038/s41418-021-00916-7",
"doi-asserted-by": "publisher",
"key": "112550_B117"
},
{
"DOI": "10.1016/S2213-2600(21)00222-8",
"doi-asserted-by": "publisher",
"key": "112550_B118"
},
{
"DOI": "10.1056/NEJMoa1912388",
"doi-asserted-by": "publisher",
"key": "112550_B119"
},
{
"DOI": "10.7326/M20-3742",
"doi-asserted-by": "publisher",
"key": "112550_B120"
},
{
"DOI": "10.1083/jcb.25.1.145",
"doi-asserted-by": "publisher",
"key": "112550_B121"
},
{
"DOI": "10.1016/j.semarthrit.2008.08.006",
"doi-asserted-by": "publisher",
"key": "112550_B122"
},
{
"DOI": "10.1002/art.27327",
"doi-asserted-by": "publisher",
"key": "112550_B123"
},
{
"DOI": "10.1016/S1473-3099(20)30196-1",
"doi-asserted-by": "publisher",
"key": "112550_B124"
},
{
"DOI": "10.21203/rs.3.rs-1612290/v1",
"doi-asserted-by": "publisher",
"key": "112550_B125"
},
{
"DOI": "10.34172/ipp.2021.12",
"doi-asserted-by": "publisher",
"key": "112550_B126"
},
{
"DOI": "10.1136/jim-2020-001747",
"doi-asserted-by": "publisher",
"key": "112550_B127"
},
{
"DOI": "10.1161/JAHA.120.018993",
"doi-asserted-by": "publisher",
"key": "112550_B128"
},
{
"DOI": "10.3390/jcm12010142",
"doi-asserted-by": "publisher",
"key": "112550_B129"
},
{
"DOI": "10.1016/j.rmed.2021.106322",
"doi-asserted-by": "publisher",
"key": "112550_B130"
},
{
"DOI": "10.1016/S0140-6736(70)92206-3",
"doi-asserted-by": "publisher",
"key": "112550_B131"
},
{
"DOI": "10.1001/jama.2020.1585",
"doi-asserted-by": "publisher",
"key": "112550_B132"
},
{
"DOI": "10.3390/ijms23031351",
"doi-asserted-by": "publisher",
"key": "112550_B133"
},
{
"DOI": "10.1016/S0140-6736(22)00519-0",
"doi-asserted-by": "publisher",
"key": "112550_B134"
},
{
"DOI": "10.1056/NEJMoa2023184",
"doi-asserted-by": "publisher",
"key": "112550_B135"
},
{
"DOI": "10.1038/s41586-020-2196-x",
"doi-asserted-by": "publisher",
"key": "112550_B136"
},
{
"DOI": "10.21037/atm.2019.09.28",
"doi-asserted-by": "publisher",
"key": "112550_B137"
},
{
"DOI": "10.1126/science.abb2507",
"doi-asserted-by": "publisher",
"key": "112550_B138"
},
{
"DOI": "10.1001/jama.2020.2648",
"doi-asserted-by": "publisher",
"key": "112550_B139"
},
{
"DOI": "10.1016/j.virol.2022.01.003",
"doi-asserted-by": "publisher",
"key": "112550_B140"
},
{
"DOI": "10.1016/S2213-2600(20)30076-X",
"doi-asserted-by": "publisher",
"key": "112550_B141"
},
{
"DOI": "10.1002/art.20317",
"doi-asserted-by": "publisher",
"key": "112550_B142"
},
{
"DOI": "10.1007/s00296-017-3823-1",
"doi-asserted-by": "publisher",
"key": "112550_B143"
},
{
"DOI": "10.1186/s43556-022-00077-0",
"doi-asserted-by": "publisher",
"key": "112550_B144"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105954",
"doi-asserted-by": "publisher",
"key": "112550_B145"
},
{
"DOI": "10.1111/all.14238",
"doi-asserted-by": "publisher",
"key": "112550_B146"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105982",
"doi-asserted-by": "publisher",
"key": "112550_B147"
},
{
"DOI": "10.1016/j.cytogfr.2021.06.002",
"doi-asserted-by": "publisher",
"key": "112550_B148"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"doi-asserted-by": "publisher",
"key": "112550_B149"
},
{
"DOI": "10.1038/s41586-020-2012-7",
"doi-asserted-by": "publisher",
"key": "112550_B150"
}
],
"reference-count": 150,
"references-count": 150,
"relation": {},
"resource": {
"primary": {
"URL": "https://pharmacia.pensoft.net/article/112550/"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Comparison of treatment of COVID-19 with inhaled bromhexine, higher doses of colchicine and hymecromone with WHO-recommended paxlovid, molnupiravir, remdesivir, anti-IL-6 receptor antibodies and baricitinib",
"type": "journal-article",
"volume": "70"
}
mitev3
