Prevention and Treatment of COVID-19 and Influenza with Bromhexine and High Doses of Colchicine
, V., MDPI AG, doi:10.20944/preprints202504.1220.v1, Apr 2025
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Review of bromhexine and colchicine for COVID-19 and influenza prevention and treatment.
See Mitev et al. for another review covering colchicine for COVID-19.
Review covers colchicine and bromhexine.
Mitev et al., 15 Apr 2025, preprint, 1 author.
Contact: vmitev@mu-sofia.bg.
Prevention and Treatment of COVID-19 and Influenza with Bromhexine and High Doses of Colchicine
doi:10.20944/preprints202504.1220.v1
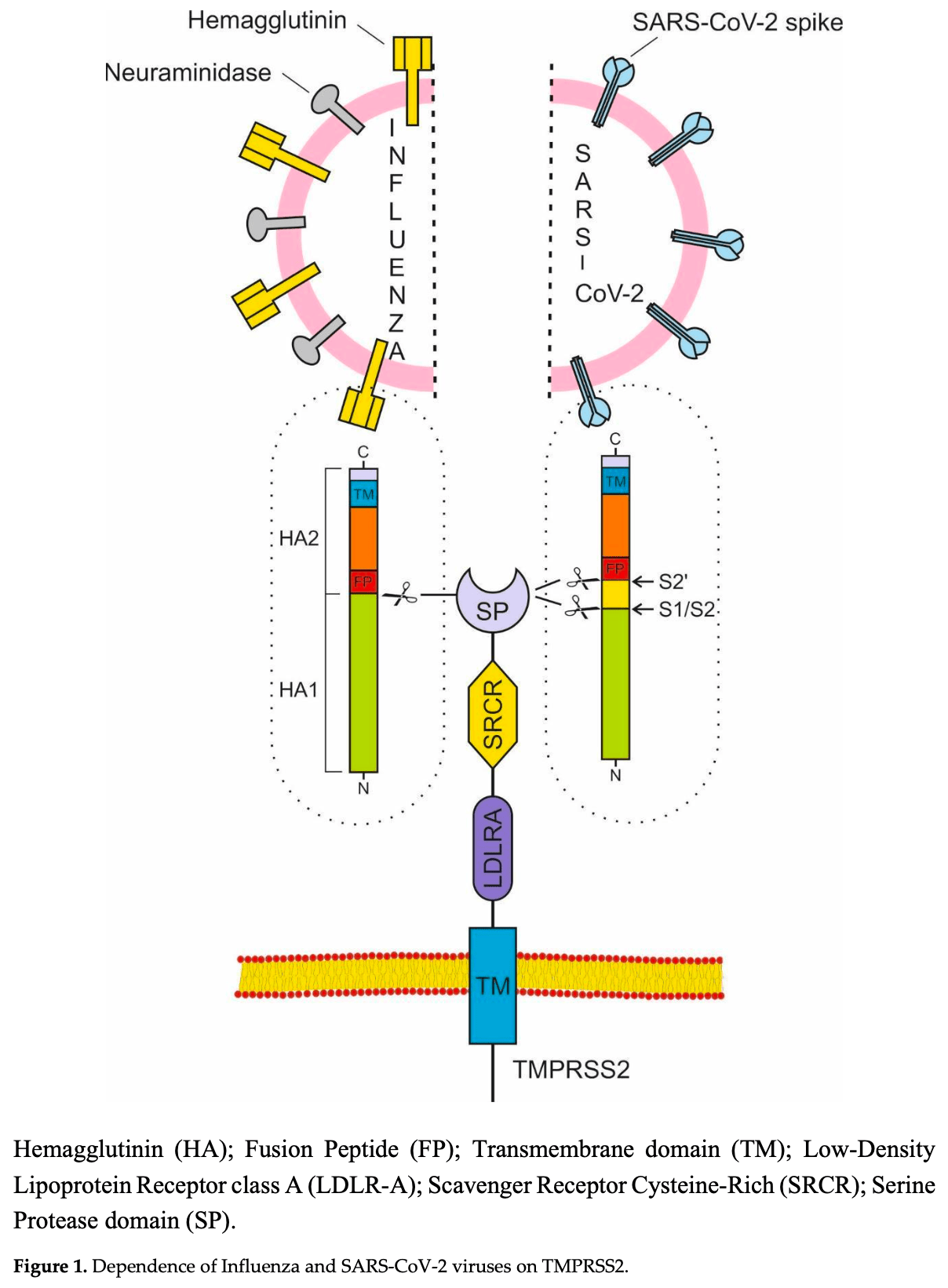
The Transmembrane Protease Serine S1 subtype 2 (TMPRSS2) and NOD-like receptor protein 3 (NLRP3) inflammasome are the main targets for the prevention and treatment of COVID-19 and influenza. The TMPRSS2 is responsible for the penetration of the SARS-CoV-2 and influenza viruses into the cell, while the hyperactivation of the NLRP3 inflammasome can lead to a cytokine storm, multiorgan damage and death. The correct strategy for preventing illness from COVID-19 and influenza is to preemptively block the TMPRSS2. Preventing the cytokine storm in COVID-19 and influenza is only effective when inhibiting NLRP3 inflammasome. Long-term prophylaxis with the TMPRSS2 inhibitor bromhexine hydrochloride and treatment with high doses of colchicine, able to inhibit the NLRP3 inflammasome, is a very effective, safe and inexpensive method against the spread and complications of COVID-19 and influenza.
References
Abe, Tahara, Sakai, Yamaguchi, Kanou et al., TMPRSS2 Is an Activating Protease for Respiratory Parainfluenza Viruses, Journal of Virology
Adam, The pandemic's true death toll: Millions more than official counts, Nature [Internet
Akbal, Dernst, Lovotti, Mangan, Mcmanus et al., How Location and Cellular Signaling Combine to Activate the NLRP3 Inflammasome, Cellular & Molecular Immunology
Allen, Scull, Moore, Holl, Mcelvania-Tekippe et al., The NLRP3 Inflammasome Mediates in Vivo Innate Immunity to Influenza a Virus through Recognition of Viral RNA, Immunity
Ambrożek-Latecka, Kozlowski, Hoser, Bandyszewska, Hanusek et al., SARS-CoV-2 and Its ORF3a, E and M Viroporins Activate Inflammasome in Human Macrophages and Induce of IL-1α in Pulmonary Epithelial and Endothelial Cells, Cell death discovery
Ansarin, Tolouian, Ardalan, Taghizadieh, Varshochi et al., Effect of Bromhexine on Clinical Outcomes and Mortality in COVID-19 patients: a Randomized Clinical Trial, BioImpacts
Barros, Nencioni, Thimoteo, Perea, Fuzaro et al., TMPRSS2 as a Key Player in Viral Pathogenesis: Influenza and Coronaviruses, Biomolecules
Bauernfeind, Horvath, Stutz, Alnemri, Macdonald et al., Cutting edge: NF-kappaB Activating Pattern Recognition and Cytokine Receptors License NLRP3 Inflammasome Activation by Regulating NLRP3 Expression, Journal of Immunology
Beesetti, Ubiquitin Ligases in Control: Regulating NLRP3 Inflammasome Activation, Frontiers in Bioscience-Landmark
Bi, Yang, Wang, Ran, Gao, Ecology and Evolution of Avian Influenza Viruses, Current Biology
Böttchere, Beyerle, Klenk, Garten, Matrosovich, Proteolytic Activation of Influenza Viruses by Serine Proteases TMPRSS2 and HAT from Human Airway Epithelium, Journal of Virology
Breining, Frølund, Højen, Gunst, Staerke et al., Camostat Mesylate against SARS-CoV-2 and COVID-19-Rationale, Dosing and Safety, Basic & Clinical Pharmacology & Toxicology
Bugge, Antalis, Wu, Type II Transmembrane Serine Proteases, Journal of Biological Chemistry
Bulanov, Yonkov, Arabadzhieva, Mitev, Successful Treatment with High-Dose Colchicine of a 101-Year-Old Patient Diagnosed with COVID-19 after an Emergency Cholecystectomy, Cureus
Böttcher-Friebertshäuser, Garten, Matrosovich, Klenk, The Hemagglutinin: A Determinant of Pathogenicity, Influenza Pathogenesis and Control
Cai, Zhao, Zhang, Liu, Ma et al., USP5 Attenuates NLRP3 Inflammasome Activation by Promoting Autophagic Degradation of NLRP3, Autophagy
Cera, Serine Proteases, IUBMB Life
Cerato, Silva, Porto, Breaking Bad: Inflammasome Activation by Respiratory Viruses, Biology
Chen, Zheng, Liu, Yan, Xu et al., Plasma CRP Level Is Positively Associated with the Severity of COVID-19, Annals of Clinical Microbiology and Antimicrobials
Chupp, Spichler-Moffarah, Søgaard, Esserman, Dziura et al., A Phase 2 Randomized, Double-Blind, Placebo-controlled Trial of Oral Camostat Mesylate for Early Treatment of COVID-19 Outpatients Showed Shorter Illness Course and Attenuation of Loss of Smell and Taste, medRxiv: The Preprint Server for Health Sciences
Dadkhah, Sharifi, The NLRP3 inflammasome: Mechanisms of activation, regulation, and Role in Diseases, International Reviews of Immunology
Depfenhart, A SARS-CoV-2 Prophylactic and Treatment; a Counter Argument against the Sole Use of Chloroquine, American Journal of Biomedical Science & Research
Depfenhart, De Villiers, Lemperle, Meyer, Somma, Potential New Treatment Strategies for COVID-19: Is There a Role for Bromhexine as add-on therapy? Internal and Emergency Medicine
Donaldson, Hirsh, Li, Holloway, Chao et al., Regulation of the Epithelial Channel by Serine Proteases in Human Airways, Journal of Biological Chemistry
Du, Kao, Zhou, He, Zhao et al., Cleavage of Spike Protein of SARS Coronavirus by Protease Factor Xa Is Associated with Viral Infectivity, Biochemical and Biophysical Research Communications
Duan, Wang, Cai, Kelley, He, The leucine-rich Repeat (LRR) Domain of NLRP3 Is Required for NLRP3 Inflammasome Activation in Macrophages, Journal of Biological Chemistry
Eisfeld, Simonis, Winter, Chhen, Ströh et al., Viral Glycoproteins Induce NLRP3 Inflammasome Activation and Pyroptosis in Macrophages, Viruses
Esumi, Ishibashi, Yamaguchi, Nakajima, Tai et al., Transmembrane Serine Protease TMPRSS2 Activates Hepatitis C Virus Infection, Hepatology
Franchi, Eigenbrod, Núñez, Cutting Edge: TNF-α Mediates Sensitization to ATP and Silica via the NLRP3 Inflammasome in the Absence of Microbial Stimulation, The Journal of Immunology
Fraser, Beldar, Seitova, Hutchinson, Mannar et al., Structure and activity of human TMPRSS2 protease implicated in SARS-CoV-2 activation, Nature Chemical Biology
Fu, Zheng, Zhou, Tang, Chen et al., Re-recognizing Bromhexine hydrochloride: Pharmaceutical Properties and Its Possible Role in Treating Pediatric COVID-19, European Journal of Clinical Pharmacology
Ghayour, Nazari, Keramat, Shahbazi, Eslami-Ghayour, Evaluation of the Efficacy of Nacetylcysteine and Bromhexine Compared with Standard Care in Preventing Hospitalization of Outpatients with COVID-19: a Double Blind Randomized Clinical Trial, Revista Clínica Española
Guarda, Braun, Staehli, Tardivel, Mattmann et al., Type I Interferon Inhibits Interleukin-1 Production and Inflammasome Activation, Immunity
Guarnieri, Angelin, Murdock, Schaefer, Portluri et al., SARS-COV-2 Viroporins Activate the NLRP3-inflammasome by the Mitochondrial Permeability Transition Pore, Frontiers in Immunology
Hamamoto, Kawamura, Uchida, Hiramatsu, Katori et al., Increased ACE2 and TMPRSS2 Expression in Ulcerative Colitis, Pathology -Research and Practice
Harbig, Mernberger, Bittel, Pleschka, Schughart et al., Transcriptome profiling and protease inhibition experiments identify proteases that activate H3N2 influenza A and influenza B viruses in murine airways
Hoang, Nguyen, Tran, Genetic Susceptibility of ACE2 and TMPRSS2 in Six Common Cancers and Possible Impacts on COVID-19, Cancer Research and Treatment
Hoffmann, Kleine-Weber, Pöhlmann, A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells, Molecular Cell
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell
Ichinohe, Lee, Ogura, Flavell, Iwasaki, Inflammasome Recognition of Influenza Virus Is Essential for Adaptive Immune Responses, The Journal of Experimental Medicine
Iwata-Yoshikawa, Okamura, Shimizu, Hasegawa, Takeda et al., TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection, Journal of Virology
Ji, Song, He, Guo, Chen et al., NIMA-related Kinase 7 Amplifies NLRP3 Inflammasome pro-inflammatory Signaling in microglia/macrophages and Mice Models of Spinal Cord Injury, Experimental Cell Research
Johnson, Mueller, Updating the accounts: Global mortality of the 1918-1920 "spanish" influenza pandemic, Bulletin of the History of Medicine
Kelleni, SARS CoV-2 Viral Load Might Not Be the Right Predictor of COVID-19 Mortality, Journal of Infection
Kelley, Jeltema, Duan, He, The NLRP3 Inflammasome: an Overview of Mechanisms of Activation and Regulation, International Journal of Molecular Sciences
Khan, Mubariz, Khlidj, Nasir, Ramadan et al., Safety and Efficacy of Camostat Mesylate for Covid-19: a Systematic Review and Meta-analysis of Randomized Controlled Trials, BMC Infectious Diseases
Kim, Heinlein, Hackman, Nelson, Phenotypic Analysis of Mice Lacking the Tmprss2-Encoded Protease, Molecular and Cellular Biology
Kim, The Mechanism of the NLRP3 Inflammasome Activation and Pathogenic Implication in the Pathogenesis of Gout, Journal of Rheumatic Diseases
Kinoshita, Shinoda, Nishizaki, Shiraki, Hirai et al., A multicenter, double-blind, randomized, parallel-group, placebo-controlled Study to Evaluate the Efficacy and Safety of Camostat Mesilate in Patients with COVID-19 (CANDLE study), BMC Medicine
Koch, Uckeley, Doldan, Stanifer, Boulant et al., TMPRSS2 Expression Dictates the Entry Route Used by SARS-CoV-2 to Infect Host Cells, The EMBO Journal
Krammer, Smith, Fouchier, Peiris, Kedzierska et al., Influenza. Nature Reviews Disease Primers, Internet
Lee, Shin, The Type I Interferon Response in COVID-19: Implications for Treatment, Nature Reviews Immunology
Li, He, Luo, Zheng, Zhou et al., Paeniclostridium sordellii hemorrhagic toxin targets TMPRSS2 to induce colonic epithelial lesions, Nature Communications
Lilov, Palaveev, Mitev, High Doses of Colchicine Act as "Silver Bullets" against Severe COVID-19, Cureus
Limburg, Harbig, Bestle, Stein, Moulton et al., TMPRSS2 Is the Major Activating Protease of Influenza a Virus in Primary Human Airway Cells and Influenza B Virus in Human Type II Pneumocytes, Journal of Virology
Liu, Wang, Shi, Association between IL-6 and Severe Disease and Mortality in COVID-19 disease: a Systematic Review and meta-analysis, Postgraduate Medical Journal
Maggio, Corsini, Repurposing the Mucolytic Cough Suppressant and TMPRSS2 Protease Inhibitor Bromhexine for the Prevention and Management of SARS-CoV-2 Infection, Pharmacological Research
Manson, Crooks, Naja, Ledlie, Goulden et al., COVID-19-associated Hyperinflammation and Escalation of Patient care: a Retrospective Longitudinal Cohort Study, The Lancet Rheumatology
Marinov, Mondeshki, Georgiev, Dimitrova, Mitev, Effects of long-term Prophylaxis with Bromhexine Hydrochloride and Treatment with High Colchicine Doses of COVID-19, Pharmacia
Martens, Van Loo, A20 at the Crossroads of Cell Death, Inflammation, and Autoimmunity, Cold Spring Harbor Perspectives in Biology
Masters, Mielke, Cornish, Sutton, Donnell et al., Regulation of interleukin-1beta by interferon-gamma Is Species specific, Limited by Suppressor of Cytokine Signalling 1 and Influences Production, EMBO Reports
Mckee, Fischer, Bezbradica, Coll, PHOrming the inflammasome: Phosphorylation Is a Critical Switch in Inflammasome Signalling, Biochemical Society Transactions
Melis, Diaz, Kleiner, Zamboni, Kabat et al., Viral Expression and Molecular Profiling in Liver Tissue versus Microdissected Hepatocytes in Hepatitis B Virus -Associated Hepatocellular Carcinoma, Journal of translational medicine
Mitev, Colchicine-The Divine Medicine against COVID-19, Journal of Personalized Medicine
Mitev, Comparison of Treatment of COVID-19 with Inhaled bromhexine, Higher Doses of Colchicine and Hymecromone with WHO-recommended paxlovid, molnupiravir, remdesivir, anti-IL-6 Receptor Antibodies and Baricitinib, Pharmacia/Farmaciâ
Mitev, High colchicine doses are really silver bullets against COVID-19, Acta Medica Bulgarica
Mitev, Marinov, Tiholov, Tashkov, Bilyukov et al., High Colchicine Doses Are More Effective in COVID-19 Outpatients than Nirmatrelvir/Ritonavir, Remdesivir, and Molnupiravir
Mitev, Mitev, Prevention and Treatment of COVID-19 and Influenza with Bromhexine and Colchicine
Mitev, Mondeshki, Miteva, Tashkov, Dimitrova, COVID-19 Prophylactic Effect of Bromhexine Hydrochloride
Mitev, Mondeshki, Miteva, Tashkov, Dimitrova, COVID-19 Prophylactic Effect of Bromhexine Hydrochloride, Preprints
Mitev, Tsanko, Marinov, Bilyukov, Colchicine, Bromhexine, and Hymecromone as Part of COVID-19 Treatment-Cold, Warm, doi:10.9734/bpi/codhr/v10/5310A
Mondeshki, Bilyukov, Mitev, Effect of an Accidental Colchicine Overdose in a COVID-19 Inpatient with Bilateral Pneumonia and Pericardial Effusion, Cureus
Mondeshki, Bilyukov, Tomov, Mihaylov, Mitev, Complete, Rapid Resolution of Severe Bilateral Pneumonia and Acute Respiratory Distress Syndrome in a COVID-19 Patient: Role for a Unique Therapeutic Combination of Inhalations with Bromhexine, Higher Doses of Colchicine, and Hymecromone, Cureus
Mondeshki, Mitev, High-Dose Colchicine: Key Factor in the Treatment of Morbidly Obese COVID-19 Patients, Cureus
Mykytyn, Breugem, Geurts, Beumer, Schipper et al., SARS-CoV-2 Omicron Entry Is Type II Transmembrane Serine protease-mediated in Human Airway and Intestinal Organoid Models, Journal of virology
Nakakubo, Unoki, Kitajima, Terada, Gatanaga et al., Serum Lactate Dehydrogenase Level One Week after Admission Is the Strongest Predictor of Prognosis of COVID-19: a Large Observational Study Using the COVID-19 Registry Japan, Viruses
Ogbac, Tamayo, Effect of Bromhexine among COVID-19 Patients -a Meta-anaylsis, 대한결핵및호흡기학회 추계학술발표초록집
Ou, Liu, Lei, Li, Mi et al., Characterization of Spike Glycoprotein of SARS-CoV-2 on Virus Entry and Its Immune cross-reactivity with SARS-CoV, Nature Communications
Paik, Kim, Silwal, Sasakawa, An Update on the Regulatory Mechanisms of NLRP3 Inflammasome Activation, Cellular & Molecular Immunology
Palazón-Riquelme, Worboys, Green, Valera, Martín-Sánchez et al., USP7 and USP47 Deubiquitinases Regulate NLRP3 Inflammasome Activation, EMBO Reports
Pan, Shen, Yu, Ge, Chen et al., SARS-CoV-2 N Protein Promotes NLRP3 Inflammasome Activation to Induce Hyperinflammation, Nature Communications
Pan, Wu, Wei, Chen, Wu et al., Structural and Functional Basis of JAMM Deubiquitinating Enzymes in Disease, Biomolecules
Planès, Pinilla, Santoni, Hessel, Passemar et al., Human NLRP1 Is a Sensor of Pathogenic Coronavirus 3CL Proteases in Lung Epithelial Cells, Molecular Cell
Potere, Buono, Caricchio, Cremer, Vecchié et al., Interleukin-1 and the NLRP3 Inflammasome in COVID-19: Pathogenetic and Therapeutic Implications, eBioMedicine
Schwerdtner, Schmacke, Nave, Limburg, Steinmetzer et al., Unveiling the Role of TMPRSS2 in the Proteolytic Activation of Pandemic and Zoonotic Influenza Viruses and Coronaviruses in Human Airway Cells, Viruses
Sefik, Qu, Junqueira, Kaffe, Mirza et al., Inflammasome Activation in Infected Macrophages Drives COVID-19 Pathology, Nature
Shen, Mao, Wu, Tanaka, Zhang, TMPRSS2: A potential target for treatment of influenza virus and coronavirus infections
Simmons, Gosalia, Rennekamp, Reeves, Diamond et al., Inhibitors of Cathepsin L Prevent Severe Acute Respiratory Syndrome Coronavirus Entry, Proceedings of the National Academy of Sciences
Simmons, Zmora, Gierer, Heurich, Pöhlmann, Proteolytic Activation of the SARS-coronavirus Spike protein: Cutting Enzymes at the Cutting Edge of Antiviral Research, Antiviral Research
Slaats, Oever, Van De Veerdonk, Netea, IL-1β/IL-6/CRP and IL-18/ferritin: Distinct Inflammatory Programs in Infections. Bliska JB, PLOS Pathogens
Smart, Polachek, COVID-19 Vaccine and risk-taking, Journal of risk and uncertainty
Song, Li, Regulation of NLRP3 Inflammasome by Phosphorylation, Frontiers in Immunology
Song, Liu, Huai, Yu, Wang et al., The E3 Ubiquitin Ligase TRIM31 Attenuates NLRP3 Inflammasome Activation by Promoting Proteasomal Degradation of NLRP3, Nature Communications
Song, Liu, Xue, Bai, Wang et al., NLRP3 Phosphorylation Is an Essential Priming Event for Inflammasome Activation, Molecular Cell
Spalinger, Kasper, Gottier, Lang, Atrott et al., NLRP3 Tyrosine Phosphorylation Is Controlled by Protein Tyrosine Phosphatase PTPN22, Journal of Clinical Investigation
Sun, Liu, Huang, Xu, Hu et al., SARS-CoV-2 non-structural Protein 6 Triggers NLRP3dependent Pyroptosis by Targeting ATP6AP1, Cell Death & Differentiation
Sungnak, Huang, Bécavin, Berg, Queen et al., SARS-CoV-2 Entry Factors Are Highly Expressed in Nasal Epithelial Cells Together with Innate Immune Genes, Nature Medicine
Sure, Bertog, Afonso, Diakov, Rinke et al., Transmembrane serine protease 2 (TMPRSS2) proteolytically activates the epithelial sodium channel (ENaC) by cleaving the channel's γ-subunit, Apr
Swanson, Deng, Ting, The NLRP3 inflammasome: Molecular Activation and Regulation to Therapeutics, Nature Reviews Immunology
Tandefelt, Boormans, Hermans, Trapman, ETS Fusion Genes in Prostate Cancer, Endocrine-Related Cancer
Tate, Mansell, An Update on the NLRP3 Inflammasome and influenza: the Road to Redemption or perdition?, Current Opinion in Immunology
Tate, Ong, Dowling, Mcauley, Robertson et al., Reassessing the Role of the NLRP3 Inflammasome during Pathogenic Influenza a Virus Infection via Temporal Inhibition, Scientific Reports
Theobald, Simonis, Georgomanolis, Kreer, Zehner et al., Long-lived Macrophage Reprogramming Drives Spike Protein-mediated Inflammasome Activation in COVID-19
Tiholov, Lilov, Georgieva, Palaveev, Tashkov et al., Effect of Increasing Doses of Colchicine on the Treatment of 333 COVID-19 Inpatients, Biotechnology & Biotechnological Equipment
Tobback, Degroote, Buysse, Delesie, Van Dooren et al., Efficacy and Safety of Camostat Mesylate in Early COVID-19 Disease in an Ambulatory setting: a Randomized placebo-controlled Phase II Trial, International Journal of Infectious Diseases
Tolouian, Moradi, Mulla, Ziaie, Haghighi et al., Bromhexine, for Post Exposure COVID-19 prophylaxis: a Randomized, Double-Blind, Placebo Control Trial
Tolouian, Mulla, Controversy with Bromhexine in COVID-19; Where We Stand, Immunopathologia Persa
Tosato, Jones, Interleukin-1 Induces interleukin-6 Production in Peripheral Blood Monocytes, Blood
Vila Méndez, Sanz, García, Del, Malo et al., Efficacy of Bromhexine versus Standard of Care in Reducing Viral Load in Patients with Mild-to-Moderate COVID-19 Disease Attended in Primary Care: a Randomized Open-Label Trial, Journal of Clinical Medicine
Vora, Lieberman, Wu, Inflammasome Activation at the Crux of Severe COVID-19, Nature Reviews Immunology
Wang, Wang, Zhang, Hu, Zhi et al., Significance of the TMPRSS2:ERG Gene Fusion in Prostate Cancer, Molecular Medicine Reports
Wang, Zhan, Zhu, Hou, Liu et al., Retrospective Multicenter Cohort Study Shows Early Interferon Therapy Is Associated with Favorable Clinical Responses in COVID-19 Patients, Cell Host & Microbe
Xu, Akinyemi, Chitre, Loeb, Lednicky et al., SARS-CoV-2 Viroporin Encoded by ORF3a Triggers the NLRP3 Inflammatory Pathway, Virology
Yang, Wang, Kouadir, Song, Shi, Recent Advances in the Mechanisms of NLRP3 Inflammasome Activation and Its Inhibitors, Cell Death & Disease
Yin, Marrone, Peace, Neill, NLRP3, the Inflammasome and COVID-19 Infection, QJM: An International Journal of Medicine
Zhang, Fang, Wang, Li, Cheng et al., Applicability of a Sensitive Duplex real-time PCR Assay for Identifying B/Yamagata and B/Victoria Lineages of Influenza Virus from Clinical Specimens, Applied Microbiology and Biotechnology
Zhang, Li, The Emerging Roles of Pellino Family in Pattern Recognition Receptor Signaling, Frontiers in Immunology
Zhang, Meszaros, He, Xu, De et al., Protein Kinase D at the Golgi Controls NLRP3 Inflammasome Activation, The Journal of Experimental Medicine
DOI record:
{
"DOI": "10.20944/preprints202504.1220.v1",
"URL": "http://dx.doi.org/10.20944/preprints202504.1220.v1",
"abstract": "<jats:p>The Transmembrane Protease Serine S1 subtype 2 (TMPRSS2) and NOD-like receptor protein 3 (NLRP3) inflammasome are the main targets for the prevention and treatment of COVID-19 and influenza. The TMPRSS2 is responsible for the penetration of the SARS-CoV-2 and influenza viruses into the cell, while the hyperactivation of the NLRP3 inflammasome can lead to a cytokine storm, multiorgan damage and death. The correct strategy for preventing illness from COVID-19 and influenza is to preemptively block the TMPRSS2. Preventing the cytokine storm in COVID-19 and influenza is only effective when inhibiting NLRP3 inflammasome. Long-term prophylaxis with the TMPRSS2 inhibitor bromhexine hydrochloride and treatment with high doses of colchicine, able to inhibit the NLRP3 inflammasome, is a very effective, safe and inexpensive method against the spread and complications of COVID-19 and influenza.</jats:p>",
"accepted": {
"date-parts": [
[
2025,
4,
14
]
]
},
"author": [
{
"ORCID": "https://orcid.org/0000-0001-7528-590X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mitev",
"given": "Vanyo",
"sequence": "first"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
4,
18
]
],
"date-time": "2025-04-18T03:50:12Z",
"timestamp": 1744948212000
},
"deposited": {
"date-parts": [
[
2025,
4,
18
]
],
"date-time": "2025-04-18T03:51:56Z",
"timestamp": 1744948316000
},
"group-title": "Medicine and Pharmacology",
"indexed": {
"date-parts": [
[
2025,
4,
19
]
],
"date-time": "2025-04-19T04:04:20Z",
"timestamp": 1745035460862,
"version": "3.40.4"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
4,
15
]
]
},
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
4,
15
]
],
"date-time": "2025-04-15T00:00:00Z",
"timestamp": 1744675200000
}
}
],
"member": "1968",
"original-title": [],
"posted": {
"date-parts": [
[
2025,
4,
15
]
]
},
"prefix": "10.20944",
"published": {
"date-parts": [
[
2025,
4,
15
]
]
},
"publisher": "MDPI AG",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.preprints.org/manuscript/202504.1220/v1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "Prevention and Treatment of COVID-19 and Influenza with Bromhexine and High Doses of Colchicine",
"type": "posted-content"
}
