Remdesivir for the treatment of COVID-19 disease: A retrospective comparative study of patients treated with and without Remdesivir
et al., medRxiv, doi:10.1101/2021.07.15.21260600, Jul 2021
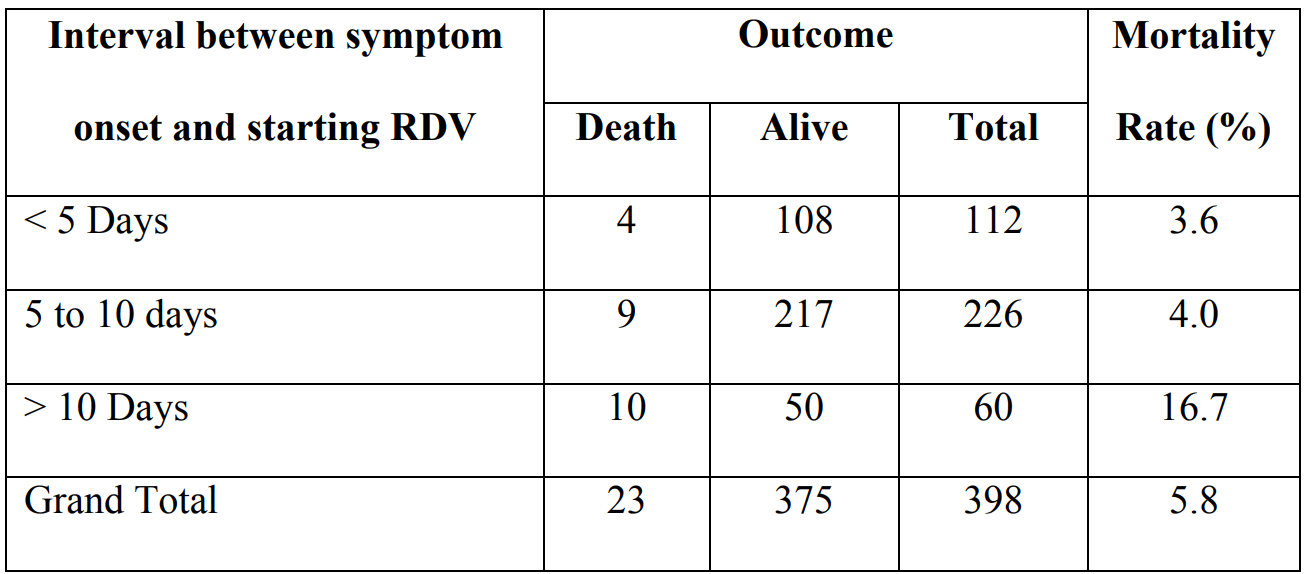
Retrospective 1,262 hospitalized patients, 398 treated with remdesivir, showing unadjusted lower mortality with treatment, and a treatment delay-response relationship.
Results for early treatment are listed separately1.
Gérard, Zhou, Wu, Kamo, Choi, Kim show increased risk of acute kidney injury, Leo, Briciu, Muntean, Petrov show increased risk of liver injury, and Negru, Cheng, Mohammed, Kwok show increased risk of cardiac disorders with remdesivir.
Remdesivir efficacy disappears with longer
followup. Mixed-effects meta-regression of efficacy as a function of
followup duration across all remdesivir studies shows decreasing efficacy with
longer followup16. This may reflect
antiviral efficacy being offset by serious adverse effects of treatment.
This study is excluded in the after exclusion results of meta-analysis:
excessive unadjusted differences between groups.
|
risk of death, 44.4% lower, RR 0.56, p = 0.03, treatment 23 of 398 (5.8%), control 27 of 260 (10.4%), NNT 22, unadjusted.
|
|
risk of death, 65.6% lower, RR 0.34, p = 0.04, treatment 4 of 112 (3.6%), control 27 of 260 (10.4%), NNT 15, unadjusted, <5 days from onset.
|
|
risk of death, 61.7% lower, RR 0.38, p = 0.009, treatment 9 of 226 (4.0%), control 27 of 260 (10.4%), NNT 16, unadjusted, 5-10 days from onset.
|
|
risk of death, 60.5% higher, RR 1.60, p = 0.18, treatment 10 of 60 (16.7%), control 27 of 260 (10.4%), unadjusted, >10 days from onset.
|
|
risk of death, 31.0% lower, RR 0.69, p = 0.30, treatment 19 of 398 (4.8%), control 18 of 260 (6.9%), NNT 47, day 14.
|
|
risk of death, 34.7% lower, RR 0.65, p = 0.32, treatment 14 of 398 (3.5%), control 14 of 260 (5.4%), NNT 54, day 10.
|
|
risk of death, 47.7% lower, RR 0.52, p = 0.22, treatment 8 of 398 (2.0%), control 10 of 260 (3.8%), NNT 54, day 7.
|
|
risk of death, 34.7% lower, RR 0.65, p = 0.53, treatment 5 of 398 (1.3%), control 5 of 260 (1.9%), NNT 150, day 5.
|
|
risk of death, 12.9% lower, RR 0.87, p = 1.00, treatment 4 of 398 (1.0%), control 3 of 260 (1.2%), NNT 672, day 3.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Madan et al., Remdesivir for the treatment of COVID-19 disease: A retrospective comparative study of patients treated with and without Remdesivir, medRxiv, doi:10.1101/2021.07.15.21260600.
2.
Gérard et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145.
3.
Zhou et al., Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679.
4.
Wu et al., Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS, Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828.
5.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
6.
Choi et al., Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0.
7.
Kim et al., Investigating the Safety Profile of Fast‐Track COVID‐19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study, Pharmacoepidemiology and Drug Safety, doi:10.1002/pds.70043.
8.
Leo et al., Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points, Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014.
9.
Briciu et al., Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting, Pathogens, doi:10.3390/pathogens12121453.
10.
Muntean et al., Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre, Pharmaceuticals, doi:10.3390/ph17010003.
11.
Petrov et al., The Effect of Potentially Hepatotoxic Medicinal Products on Alanine Transaminase Levels in COVID-19 Patients: A Case–Control Study, Safety and Risk of Pharmacotherapy, doi:10.30895/2312-7821-2025-458.
12.
Negru et al., Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data, Biomedicines, doi:10.3390/biomedicines13061387.
13.
Cheng et al., Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase, Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z.
14.
Mohammed et al., Bradycardia associated with remdesivir treatment in coronavirus disease 2019 patients: A propensity score-matched analysis, Medicine, doi:10.1097/MD.0000000000044501.
Madan et al., 19 Jul 2021, retrospective, India, preprint, 22 authors.
Remdesivir for the treatment of COVID-19 disease: A retrospective comparative study of patients treated with and without Remdesivir
doi:10.1101/2021.07.15.21260600
Background: Remdesivir (RDV) in coronavirus disease 2019 has been found to be beneficial in patients with severe disease; however, its role in mild-moderate disease and its optimal timing need to be identified. Objective: To assess the course of illness and final outcome in patients who received RDV at various stages of illness, and compare it to the non-RDV group. Methods: This is a retrospective data analysis of 1262 COVID-19 patients hospitalized from May5, 2020 to August 31, 2020. The primary outcomes were progression to mechanical ventilation (MV) or death. Kaplan Meier survival analysis and log rank test were used for evaluating primary outcomes. Results: 398 patients comprised the RDV group and 260 patients comprised the non-RDV group. 2/3 rd of patients were above 50 years of age in both the groups and 3/4 th patients were male. Mortality rate was 5.8% in RDV group (10.4% in non-RDV group). Mortality rate was 3.6%, 4% and 16.7% when RDV was started within 5 days, 5 to 10 days and after 10 days of symptom onset respectively. Fewer patients in RDV group progressed to MV (4.0% v/s 8.2%). Earlier discharge occurred in RDV group. Use of supplemental oxygen was observed in 44.7% patients in RDV group (54.2% in non-RDV group). No significant adverse events were observed with RDV. Survival analysis showed that probability of event (death) was significant for patients with hypertension (HT) and/or diabetes mellitus (DM) in RDV group.
Conclusion: Early initiation of RDV is associated with shorter hospital stay, lower mortality as well as reduced need for supplemental oxygen and mechanical ventilation. .
References
Adamsick, Gandhi, Bidell, Remdesivir in Patients with Acute or Chronic Kidney Disease and COVID-19, J Am Soc Nephrol
Beigel, Tomashek, Dodd, Remdesivir for the Treatment of COVID-19-Final Report, N Engl J Med, doi:10.1056/NEJMoa2007764
Cantini, Goletti, Petrone, Immune therapy, or Antiviral Therapy, or Both for COVID-19; A systematic review, Drugs, doi:10.1007/s40265-020-01421-w
Ng Deborah, Chiaw Yee, Chan, Fever Patterns, Cytokine Profiles, and Outcomes in COVID-19, Open Forum Infect Dis, doi:10.1093/ofid/ofaa375.eCollection2020
Oleander, Perez, Go, Remdesivir for Severe Coronavirus Disease 2019 (COVID-19) Versus a Cohort Receiving Standard of Care, Clin Infect Dis, doi:10.1093/cid/ciaa1041
Spinner, Gottlieb, Criner, Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients with Moderate COVID-19, A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2020.16349
Thakare, Gandhi, Modi, Safety of Remdesivir in Patients With Acute Kidney Injury or CKD, Kidney Int Rep
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Wang, Zhang, Du, Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial, Lancet, doi:10.1016/S0140-6736(20)31022-9
DOI record:
{
"DOI": "10.1101/2021.07.15.21260600",
"URL": "http://dx.doi.org/10.1101/2021.07.15.21260600",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>Remdesivir (RDV) in coronavirus disease 2019 (COVID-19) has been found to be beneficial in patients with severe disease; however, its role in mild-moderate disease and its optimal timing need to be identified.</jats:p></jats:sec><jats:sec><jats:title>Objective</jats:title><jats:p>To assess the course of illness and final outcome in patients who received RDV at various stages of illness, and compare it to the non-RDV group.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>This is a retrospective data analysis of 1262 COVID-19 patients hospitalized from May5, 2020 to August 31, 2020. The primary outcomes were progression to mechanical ventilation (MV) or death. Kaplan Meier survival analysis and log rank test were used for evaluating primary outcomes.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>398 patients comprised the RDV group and 260 patients comprised the non-RDV group. 2/3<jats:sup>rd</jats:sup> of patients were above 50 years of age in both the groups and 3/4<jats:sup>th</jats:sup> patients were male. Mortality rate was 5.8% in RDV group (10.4% in non-RDV group). Mortality rate was 3.6%, 4% and 16.7% when RDV was started within 5 days, 5 to 10 days and after 10 days of symptom onset respectively. Fewer patients in RDV group progressed to MV (4.0% v/s 8.2%). Earlier discharge occurred in RDV group. Use of supplemental oxygen was observed in 44.7% patients in RDV group (54.2% in non-RDV group). No significant adverse events were observed with RDV. Survival analysis showed that probability of event (death) was significant for patients with hypertension (HT) and/or diabetes mellitus (DM) in RDV group.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>Early initiation of RDV is associated with shorter hospital stay, lower mortality as well as reduced need for supplemental oxygen and mechanical ventilation.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2021,
7,
19
]
]
},
"author": [
{
"affiliation": [],
"family": "Madan",
"given": "Surabhi",
"sequence": "first"
},
{
"affiliation": [],
"family": "Patel",
"given": "Amit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sharan",
"given": "Kartikae",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ghosh",
"given": "Shayon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Venugopal",
"given": "Vishnu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shah",
"given": "Nitesh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shah",
"given": "Bhagyesh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thakkar",
"given": "Vipul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chovatiya",
"given": "Rashmi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shah",
"given": "Hardik",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dabhi",
"given": "Pradip",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Minesh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meghnathi",
"given": "Bhowmik",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sankhla",
"given": "Vineet",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kapoor",
"given": "Vipul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Tejas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Soni",
"given": "Maulik",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bapat",
"given": "Nirav",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shah",
"given": "Kaivan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chandarana",
"given": "Ritanshu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bhatt",
"given": "Parloop",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rana",
"given": "Manish",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
7,
19
]
],
"date-time": "2021-07-19T21:20:20Z",
"timestamp": 1626729620000
},
"deposited": {
"date-parts": [
[
2021,
7,
21
]
],
"date-time": "2021-07-21T08:15:51Z",
"timestamp": 1626855351000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2022,
4,
5
]
],
"date-time": "2022-04-05T02:59:57Z",
"timestamp": 1649127597081
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2021,
7,
19
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.07.15.21260600",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
7,
19
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
7,
19
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"key": "2021072101150660000_2021.07.15.21260600v1.1",
"unstructured": "Weekly epidemiological update on COVID-19 - 6 July 2021, Edition 47, 6 July 2021, Emergency Situational Updates, Weekly epidemiological update on COVID-19 - 6 July 2021 (who.int)"
},
{
"DOI": "10.1007/s40265-020-01421-w",
"doi-asserted-by": "crossref",
"key": "2021072101150660000_2021.07.15.21260600v1.2",
"unstructured": "Cantini F , Goletti D , Petrone L et al. Immune therapy, or Antiviral Therapy, or Both for COVID-19; A systematic review. Drugs, October 2020.https://doi.org/10.1007/s40265-020-01421-w"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"doi-asserted-by": "publisher",
"key": "2021072101150660000_2021.07.15.21260600v1.3"
},
{
"key": "2021072101150660000_2021.07.15.21260600v1.4",
"unstructured": "FDA’ s approval of Veklury (remdesivir) for the treatment of COVID-19 – The Science of Safety and Effectiveness. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fdas-approval-veklury-remdesivir-treatment-covid-19-science-safety-and-effectiveness"
},
{
"key": "2021072101150660000_2021.07.15.21260600v1.5",
"unstructured": "World Health Organization. Therapeutics and COVID-19: living guideline. https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.2, 6 July 2021, COVID-19: Clinical care"
},
{
"key": "2021072101150660000_2021.07.15.21260600v1.6",
"unstructured": "National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. https://covid19treatmentguidelines.nih.gov/ (Accessed on May 27, 2021)."
},
{
"key": "2021072101150660000_2021.07.15.21260600v1.7",
"unstructured": "Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19 https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ (Accessed on February 21, 2021)."
},
{
"key": "2021072101150660000_2021.07.15.21260600v1.8",
"unstructured": "Clinical management protocol: COVID-19, Government of india ministry of health and family welfare, directorate general of health services, (emr division), version 5, 03.07.20."
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "2021072101150660000_2021.07.15.21260600v1.9"
},
{
"DOI": "10.1093/cid/ciaa1041",
"doi-asserted-by": "crossref",
"key": "2021072101150660000_2021.07.15.21260600v1.10",
"unstructured": "Oleander SA , Perez KK , Go AS , et al. Remdesivir for Severe Coronavirus Disease 2019 (COVID-19) Versus a Cohort Receiving Standard of Care. Clin Infect Dis 2020. Available from: https://doi.org/10.1093/cid/ciaa1041"
},
{
"DOI": "10.1001/jama.2020.16349",
"doi-asserted-by": "publisher",
"key": "2021072101150660000_2021.07.15.21260600v1.11"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"doi-asserted-by": "publisher",
"key": "2021072101150660000_2021.07.15.21260600v1.12"
},
{
"DOI": "10.1093/ofid/ofaa375",
"doi-asserted-by": "publisher",
"key": "2021072101150660000_2021.07.15.21260600v1.13"
},
{
"DOI": "10.1681/ASN.2020050589",
"doi-asserted-by": "publisher",
"key": "2021072101150660000_2021.07.15.21260600v1.14"
},
{
"DOI": "10.1016/j.ekir.2020.10.005",
"article-title": "Safety of Remdesivir in Patients With Acute Kidney Injury or CKD",
"doi-asserted-by": "crossref",
"first-page": "206",
"journal-title": "Kidney Int Rep",
"key": "2021072101150660000_2021.07.15.21260600v1.15",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2023184",
"doi-asserted-by": "publisher",
"key": "2021072101150660000_2021.07.15.21260600v1.16"
},
{
"key": "2021072101150660000_2021.07.15.21260600v1.17",
"unstructured": "Gilead’ s Veklury® (Remdesivir) Associated With a Reduction in Mortality Rate in Hospitalized Patients With COVID-19 Across Three Analyses of Large Retrospective Real-World Data Sets. Available from: Gilead’ s Veklury® (Remdesivir) Associated With a Reduction in Mortality Rate in Hospitalized Patients With COVID-19 Across Three Analyses of Large Retrospective Real-World Data Sets | Business Wire"
}
],
"reference-count": 17,
"references-count": 17,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2021.07.15.21260600"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Remdesivir for the treatment of COVID-19 disease: A retrospective comparative study of patients treated with and without Remdesivir",
"type": "posted-content"
}