Disease Progression of Hospitalized Elderly Patients with Omicron BA.2 Treated with Molnupiravir
et al., Infectious Diseases and Therapy, doi:10.1007/s40121-022-00716-7, Oct 2022
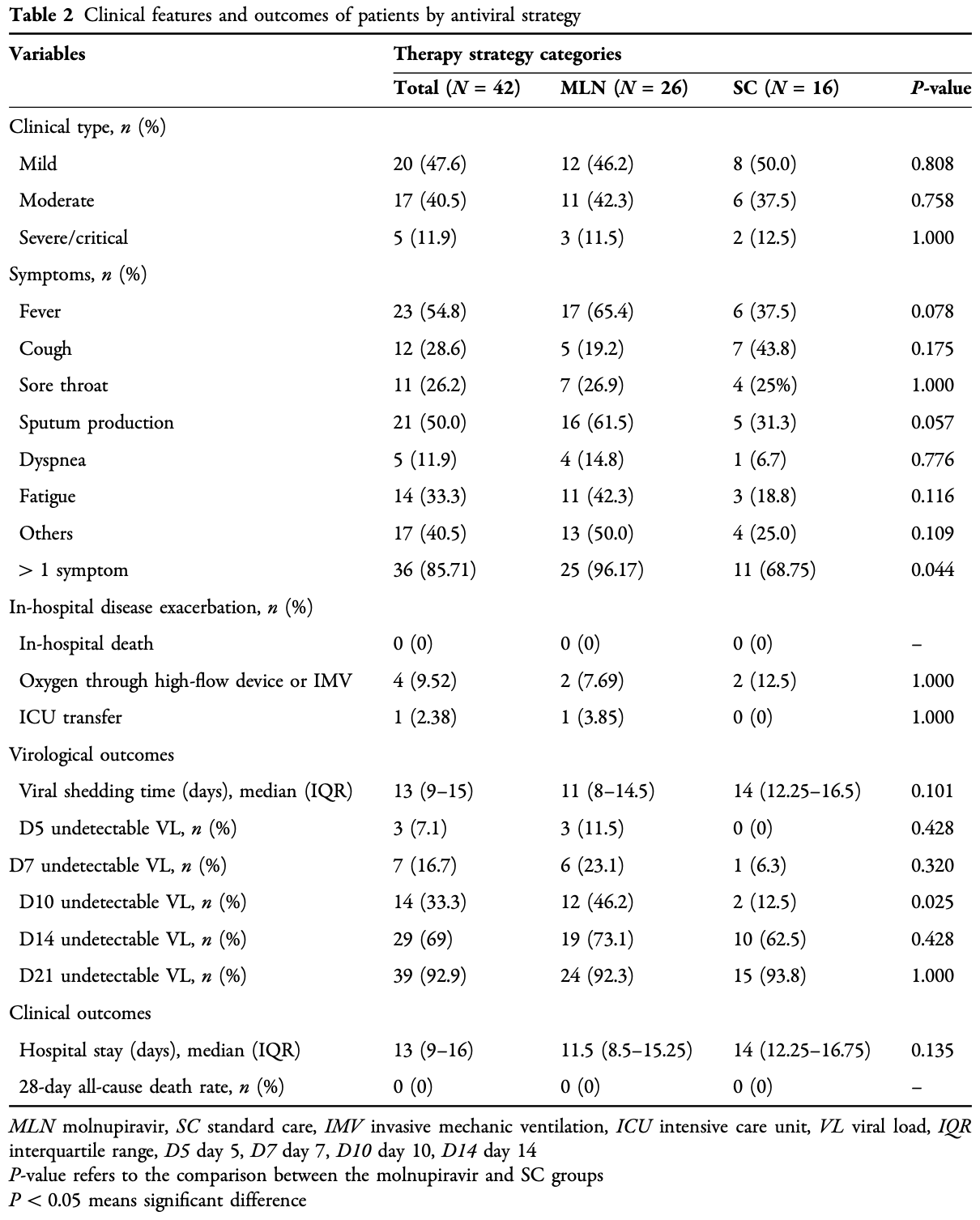
Retrospective 42 elderly patients in China showing faster viral clearance with molnupiravir.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments25.
|
risk of ICU admission, 161.5% higher, RR 2.62, p = 1.00, treatment 1 of 26 (3.8%), control 0 of 16 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
risk of progression, 7.7% lower, RR 0.92, p = 1.00, treatment 3 of 26 (11.5%), control 2 of 16 (12.5%), NNT 104, dyspnea or ICU.
|
|
hospitalization time, 17.9% lower, relative time 0.82, p = 0.14, treatment 26, control 16.
|
|
time to viral-, 21.4% lower, relative time 0.79, p = 0.10, treatment 26, control 16.
|
|
risk of no viral clearance, 23.1% higher, RR 1.23, p = 1.00, treatment 2 of 26 (7.7%), control 1 of 16 (6.2%), day 21.
|
|
risk of no viral clearance, 28.2% lower, RR 0.72, p = 0.51, treatment 7 of 26 (26.9%), control 6 of 16 (37.5%), NNT 9.5, day 14.
|
|
risk of no viral clearance, 38.5% lower, RR 0.62, p = 0.04, treatment 14 of 26 (53.8%), control 14 of 16 (87.5%), NNT 3.0, day 10.
|
|
risk of no viral clearance, 17.9% lower, RR 0.82, p = 0.22, treatment 20 of 26 (76.9%), control 15 of 16 (93.8%), NNT 5.9, day 7.
|
|
risk of no viral clearance, 11.5% lower, RR 0.88, p = 0.28, treatment 23 of 26 (88.5%), control 16 of 16 (100.0%), NNT 8.7, day 5.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
23.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Liu et al., 30 Oct 2022, retrospective, China, peer-reviewed, median age 84.0, 10 authors, study period 26 March, 2022 - 31 May, 2022.
Contact: liangxuesong2000@163.com, 452055289@qq.com, redmaples2005@163.com, 18351977696@163.com, zhongwu@bmi.ac.cn, xuaijing86@163.com, fansy@bmi.ac.cn, 1042956281@qq.com, 617943178@qq.com, yxdongxu@126.com.
Disease Progression of Hospitalized Elderly Patients with Omicron BA.2 Treated with Molnupiravir
Infectious Diseases and Therapy, doi:10.1007/s40121-022-00716-7
Introduction: The efficacy of molnupiravir (MLN) on Omicron sublineages is limited. We investigated the effectiveness of MLN in older adults diagnosed with Omicron BA.2. Methods: Data of elderly COVID-19 patients (over 60 years) admitted to Chinghai Hospital (Shanghai, China) from 26 March to 31 May 2022 were reviewed. Study outcomes were a composite of undetectable viral load (VL) and disease progression [all-cause mortality, initiation of oxygen supply through high-flow device or invasive mechanical ventilation (IMV), or intensive care unit (ICU) admission] and their individual outcomes. Results: A total of 42 elderly patients were enrolled: 26 of them received MLN, 17 (40.5%) were males, the median age was 84 years, and 12 were fully vaccinated (31.0%). Among these elderly COVID-19 patients, five (11.90%) experienced obvious dyspnea or were transferred to ICU [three MLN users (11.5%) versus two non-MLN users (12.5%)]. Compared with no MLN use, MLN use was associated with rapid undetectable VL. At day 10, MLN users achieved Yayun Liu, Lingling Ge, and Shiyong Fan are co-first authors.
Author Contributions. Yayun Liu recruited the patients and collected specimens, edited the clinical data and performed the data analysis; Lingling Ge edited the clinical data of enrolled patients; Shiyong Fan drafted the manuscript; Aijing Xu recruited the patients and treated the patients; Wang, Xu Dong, Mingxiao Xu and Wenhan Fan treated the patients; Wu Zhong conceived and designed the study; Xuesong Liang conceived and designed the study; checked the data analysis and drafted the manuscript; All authors have read and approved the final manuscript. Disclosures. Yayun Liu, Lingling Ge, Shiyong Fan, Aijing Xu, Xinyu Wang, XuDong, Mingxiao Xu, Wenhan Fan, Wu Zhong and Xuesong Liang declare that they have no competing interests. Compliance with Ethics Guidelines. This study was approved by the Ethics Committee of Changhai Hospital. (CHEC2022-111).Written informed consent was exempted from all the study participants. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Data Availability. All data and analysis results are included in this article. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. All authors have read and approved the final manuscript. Open Access. This article is licensed under a Creative Commons Attribution-Non-Commercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction..
References
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Cao, Li, Yang, The adenosine analog prodrug ATV006 is orally bioavailable and has preclinical efficacy against parental SARS-CoV-2 and variants, Sci Transl Med, doi:10.1126/scitranslmed.abm7621
Dejnirattisai, Huo, Zhou, SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses, Cell, doi:10.1016/j.cell.2021.12.046
Guo, Han, Zhang, SARS-CoV-2 omicron variant: epidemiological features, biological characteristics, and clinical significance, Front Immunol, doi:10.3389/fimmu.2022.877101
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Islam, Dhawan, Nafady, Understanding the omicron variant (B.1.1.529) of SARS-CoV-2: mutational impacts, concerns, and the possible solutions, Ann Med Surg (Lond), doi:10.1016/j.amsu.2022.103737
Mohsin, Omicron SARS-CoV-2 variant of concern: a review on its transmissibility, immune evasion, reinfection, and severity, Medicine, doi:10.1097/MD.0000000000029165
Smith, Hakim, Leung, COVID-19 mortality and vaccine coverage-Hong Kong Special Administrative Region China, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7115e1
Takashita, Kinoshita, Yamayoshi, Efficacy of antibodies and antiviral drugs against Covid-19 Omicron variant, N Engl J Med
Vangeel, Chiu, Jonghe, Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 omicron and other variants of concern, Antiviral Res, doi:10.1016/j.antiviral.2022.105252
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir against mortality, hospitalization, and in-hospital outcomes among community-dwelling, ambulatory COVID-19 patients during the BA.2.2 wave in Hong Kong: an observational study, doi:10.1101/2022.05.26.22275631
Wong, Au, Lau, Lau, Cowling et al., nirmatrelvir/ritonavir among COVID-19 inpatients during Hong Kong's Omicron BA.2 wave: an observational study, doi:10.1101/2022.05.19.22275291
DOI record:
{
"DOI": "10.1007/s40121-022-00716-7",
"ISSN": [
"2193-8229",
"2193-6382"
],
"URL": "http://dx.doi.org/10.1007/s40121-022-00716-7",
"alternative-id": [
"716"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "8 August 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "12 October 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "30 October 2022"
}
],
"author": [
{
"affiliation": [],
"family": "Liu",
"given": "Yayun",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ge",
"given": "Lingling",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fan",
"given": "Shiyong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xu",
"given": "Aijing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Xinyu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dong",
"given": "Xu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xu",
"given": "Mingxiao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fan",
"given": "Wenhan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhong",
"given": "Wu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liang",
"given": "Xuesong",
"sequence": "additional"
}
],
"container-title": "Infectious Diseases and Therapy",
"container-title-short": "Infect Dis Ther",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
10,
30
]
],
"date-time": "2022-10-30T17:02:36Z",
"timestamp": 1667149356000
},
"deposited": {
"date-parts": [
[
2022,
10,
30
]
],
"date-time": "2022-10-30T17:09:08Z",
"timestamp": 1667149748000
},
"funder": [
{
"award": [
"COVID-ZD-006",
"COVID-ZD-011"
],
"name": "Key research project of COVID-19 by Changhai hospital of Naval Medical University"
},
{
"award": [
"2021YFC2300704"
],
"name": "the National Key Research and Development Project"
}
],
"indexed": {
"date-parts": [
[
2022,
10,
31
]
],
"date-time": "2022-10-31T04:44:37Z",
"timestamp": 1667191477310
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
10,
30
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
30
]
],
"date-time": "2022-10-30T00:00:00Z",
"timestamp": 1667088000000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
30
]
],
"date-time": "2022-10-30T00:00:00Z",
"timestamp": 1667088000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40121-022-00716-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s40121-022-00716-7/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s40121-022-00716-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1007",
"published": {
"date-parts": [
[
2022,
10,
30
]
]
},
"published-online": {
"date-parts": [
[
2022,
10,
30
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/j.amsu.2022.103737",
"author": "F Islam",
"doi-asserted-by": "publisher",
"first-page": "103737",
"journal-title": "Ann Med Surg (Lond).",
"key": "716_CR1",
"unstructured": "Islam F, Dhawan M, Nafady MH, et al. Understanding the omicron variant (B.1.1.529) of SARS-CoV-2: mutational impacts, concerns, and the possible solutions. Ann Med Surg (Lond). 2022;78:103737. https://doi.org/10.1016/j.amsu.2022.103737.",
"volume": "78",
"year": "2022"
},
{
"DOI": "10.15585/mmwr.mm7115e1",
"author": "DJ Smith",
"doi-asserted-by": "publisher",
"first-page": "545",
"issue": "15",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "716_CR2",
"unstructured": "Smith DJ, Hakim AJ, Leung GM, et al. COVID-19 mortality and vaccine coverage—Hong Kong Special Administrative Region China, January 6, 2022–March 21, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(15):545–8. https://doi.org/10.15585/mmwr.mm7115e1.",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116044",
"author": "A Jayk Bernal",
"doi-asserted-by": "publisher",
"first-page": "509",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "716_CR3",
"unstructured": "Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–20. https://doi.org/10.1056/NEJMoa2116044.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2021.12.046",
"author": "W Dejnirattisai",
"doi-asserted-by": "publisher",
"first-page": "467",
"issue": "3",
"journal-title": "Cell",
"key": "716_CR4",
"unstructured": "Dejnirattisai W, Huo J, Zhou D, et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185(3):467-84e15. https://doi.org/10.1016/j.cell.2021.12.046.",
"volume": "185",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2022.877101",
"author": "Y Guo",
"doi-asserted-by": "publisher",
"first-page": "877101",
"journal-title": "Front Immunol",
"key": "716_CR5",
"unstructured": "Guo Y, Han J, Zhang Y, et al. SARS-CoV-2 omicron variant: epidemiological features, biological characteristics, and clinical significance. Front Immunol. 2022;13:877101. https://doi.org/10.3389/fimmu.2022.877101.",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1097/MD.0000000000029165",
"author": "M Mohsin",
"doi-asserted-by": "publisher",
"issue": "19",
"journal-title": "Medicine (Baltimore)",
"key": "716_CR6",
"unstructured": "Mohsin M, Mahmud S. Omicron SARS-CoV-2 variant of concern: a review on its transmissibility, immune evasion, reinfection, and severity. Medicine (Baltimore). 2022;101(19): e29165. https://doi.org/10.1097/MD.0000000000029165.",
"volume": "101",
"year": "2022"
},
{
"DOI": "10.1126/scitranslmed.abm7621",
"author": "L Cao",
"doi-asserted-by": "publisher",
"journal-title": "Sci Transl Med.",
"key": "716_CR7",
"unstructured": "Cao L, Li Y, Yang S, et al. The adenosine analog prodrug ATV006 is orally bioavailable and has preclinical efficacy against parental SARS-CoV-2 and variants. Sci Transl Med. 2022. https://doi.org/10.1126/scitranslmed.abm7621.",
"year": "2022"
},
{
"DOI": "10.1101/2022.05.19.22275291",
"author": "CKH Wong",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv.",
"key": "716_CR8",
"unstructured": "Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir among COVID-19 inpatients during Hong Kong’s Omicron BA.2 wave: an observational study. medRxiv. 2022. https://doi.org/10.1101/2022.05.19.22275291.",
"year": "2022"
},
{
"DOI": "10.1016/j.antiviral.2022.105252",
"author": "L Vangeel",
"doi-asserted-by": "publisher",
"journal-title": "Antiviral Res",
"key": "716_CR9",
"unstructured": "Vangeel L, Chiu W, De Jonghe S, et al. Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 omicron and other variants of concern. Antiviral Res. 2022;198: 105252. https://doi.org/10.1016/j.antiviral.2022.105252.",
"volume": "198",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2119407",
"author": "E Takashita",
"doi-asserted-by": "publisher",
"first-page": "995",
"issue": "10",
"journal-title": "N Engl J Med",
"key": "716_CR10",
"unstructured": "Takashita E, Kinoshita N, Yamayoshi S, et al. Efficacy of antibodies and antiviral drugs against Covid-19 Omicron variant. N Engl J Med. 2022;386(10):995–8.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1101/2022.05.26.22275631",
"author": "CKH Wong",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "716_CR11",
"unstructured": "Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir against mortality, hospitalization, and in-hospital outcomes among community-dwelling, ambulatory COVID-19 patients during the BA.2.2 wave in Hong Kong: an observational study. medRxiv. 2022. https://doi.org/10.1101/2022.05.26.22275631.",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"author": "J Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "716_CR12",
"unstructured": "Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386(15):1397–408. https://doi.org/10.1056/NEJMoa2118542.",
"volume": "386",
"year": "2022"
}
],
"reference-count": 12,
"references-count": 12,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s40121-022-00716-7"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Disease Progression of Hospitalized Elderly Patients with Omicron BA.2 Treated with Molnupiravir",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy"
}