Efficacy of late-onset antiviral treatment in immunocompromised hosts with persistent SARS-CoV-2 infection
et al., Journal of Virology, doi:10.1128/jvi.00905-24, Sep 2024 (preprint)
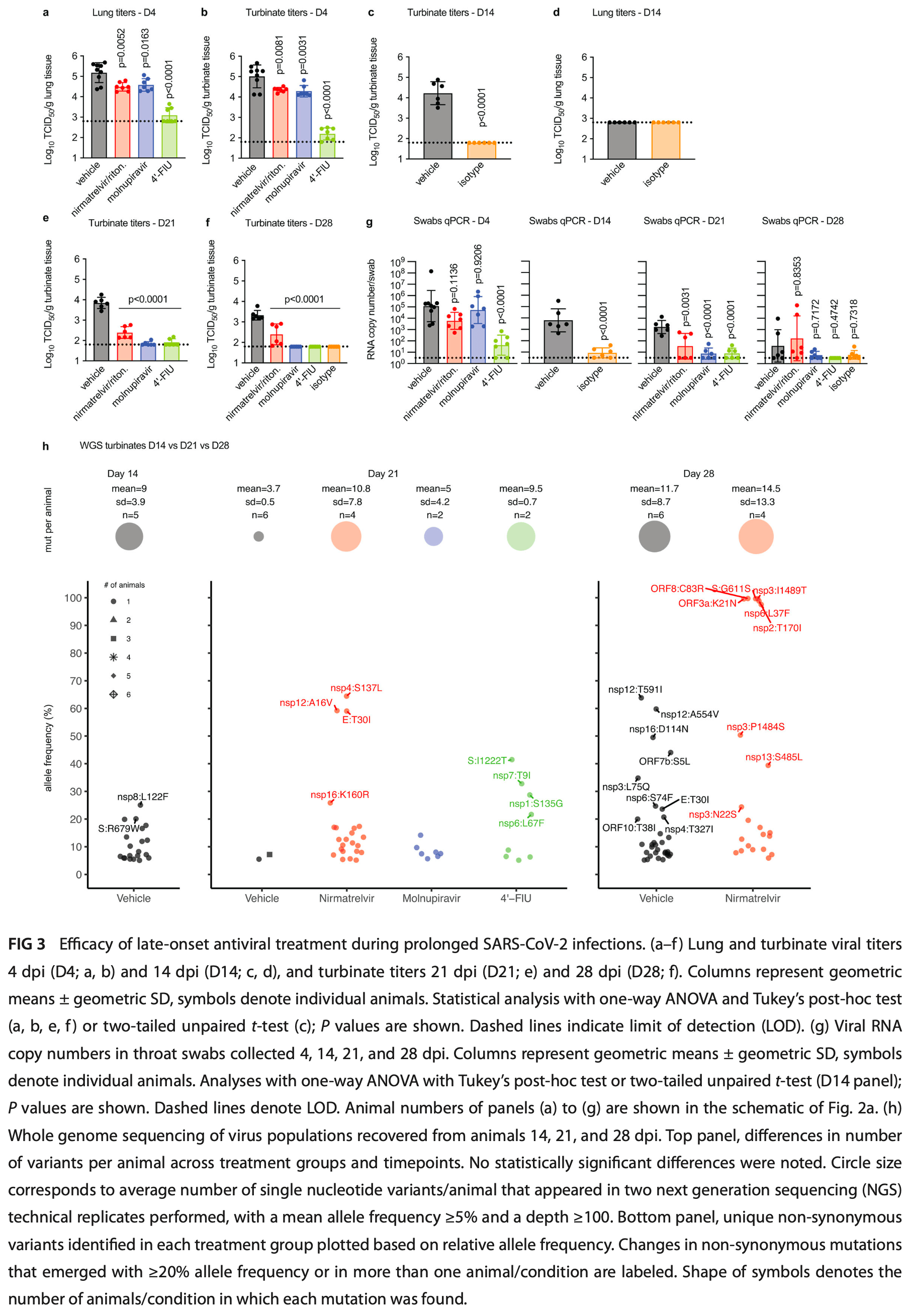
Mouse study showing that late-onset treatment with paxlovid, molnupiravir, or 4'-FlU significantly reduced persistent SARS-CoV-2 infection in immunocompromised mice, with 4'-FlU being most effective. Authors used a CD4+ and CD8+ T cell-depleted mouse model, which supported prolonged viral replication for 5 weeks. Treatment initiated 14 days post-infection lowered viral burden, but only molnupiravir and 4'-FlU were sterilizing after a 7-day regimen. Paxlovid-treated animals experienced viral rebound in the upper respiratory tract after treatment ended. A 14-day treatment course prevented rebound. The results support using direct-acting antivirals for late-onset treatment of persistent SARS-CoV-2 infection in immunocompromised hosts, but suggest treatment courses may need to be extended for maximal efficacy.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
Study covers paxlovid and molnupiravir.
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
Lieber et al., 17 Sep 2024, peer-reviewed, 11 authors.
Contact: rplemper@gsu.edu.
Efficacy of late-onset antiviral treatment in immune-compromised hosts with persistent SARS-CoV-2 infection
The immunocompromised are at high risk of prolonged SARS-CoV-2 infection and progression to severe COVID-19. However, efficacy of late-onset direct-acting antiviral (DAA) therapy with therapeutics in clinical use and experimental drugs to mitigate persistent viral replication is unclear. In this study, we employed an immunocompromised mouse model, which supports prolonged replication of SARS-CoV-2 to explore late-onset treatment options. Tandem immuno-depletion of CD4 + and CD8 + T cells in C57BL/6 mice followed by infection with SARS-CoV-2 variant of concern (VOC) beta B.1.351 resulted in prolonged infection with virus replication for five weeks after inoculation. Early-onset treatment with nirmatrelvir/ritonavir (paxlovid) or molnupiravir was only moderately efficacious, whereas the experimental therapeutic 4'-fluorourdine (4'-FlU, EIDD-2749) significantly reduced virus load in upper and lower respiratory compartments four days post infection (dpi). All antivirals significantly lowered virus burden in a 7-day treatment regimen initiated 14 dpi, but paxlovid-treated animals experienced rebound virus replication in the upper respiratory tract seven days after treatment end. Viral RNA was detectable 28 dpi in paxlovid-treated animals, albeit not in the molnupiravir or 4'-FlU groups, when treatment was initiated 14 dpi and continued for 14 days. Low-level virus replication continued 35 dpi in animals receiving vehicle but had ceased in all treatment groups. These data indicate that late-onset DAA therapy significantly shortens the duration of persistent virus replication in an immunocompromised host, which may have implications for clinical use of antiviral therapeutics to alleviate the risk of progression to severe disease in highly vulnerable patients.
Importance Four years after the onset of the global COVID-19 pandemic, the immunocompromised are at greatest risk of developing life-threatening severe disease. However, specific treatment plans for this most vulnerable patient group have not yet been developed. Employing a CD4 + and CD8 + T cell-depleted immunocompromised mouse model of SARS-CoV-2 infection, we explored therapeutic options of persistent infections with standard-of-care paxlovid, molnupiravir, and the experimental therapeutic 4'-FlU. Late-onset treatment initiated 14 days after infection was efficacious, but only 4'-FlU was rapidly sterilizing. No treatment-experienced viral variants with reduced susceptibility to the drugs emerged, albeit virus replication rebounded in animals of the paxlovid group after treatment end. This study supports the use of direct-acting antivirals for late-onset management of persistent SARS-CoV-2 infection in immunocompromised hosts. However, treatment courses likely require to be extended for maximal therapeutic benefit, calling for appropriately powered clinical trials to meet the specific needs of this patient group.
Conflict of interest MGN is a coinventor on patent WO 2019/1736002 covering composition of matter and use of 4'-FlU (EIDD-2749) and its analogs as an antiviral treatment. This study could affect his personal financial status. RKP reports contract testing from Enanta Pharmaceuticals, Atea Pharmaceuticals, and Icosagen Biosciences, and research support from Gilead Sciences, outside of the described work. ALG reports contract testing from Abbott, Cepheid, Novavax, Pfizer, Janssen and Hologic, research support from Gilead, outside of the described work. All other authors declare that they have no competing interests.
References
Abbasi, Researchers Tie Severe Immunosuppression to Chronic COVID-19 and Virus Variants, JAMA
Abdelnabi, Lassaunière, Maes, Weynand, Neyts, Comparing the Infectivity of Recent SARS-CoV-2 Omicron Sub-Variants in Syrian Hamsters, Viruses
Adler, Vidal, Langner, Vladimirova, Abdelgawad et al., An intranasal live-attenuated SARS-CoV-2 vaccine limits virus transmission, Nat Commun
Antinori, Bausch-Jurken, The Burden of COVID-19 in the Immunocompromised Patient: Implications for Vaccination and Needs for the Future, J Infect Dis
Arce, Costoya, SARS-CoV-2 infection in K18-ACE2 transgenic mice replicates human pulmonary disease in COVID-19, Cellular & Molecular Immunology
Belsky, Tullius, Lamb, Sayegh, Stanek et al., COVID-19 in immunocompromised patients: A systematic review of cancer, hematopoietic cell and solid organ transplant patients, Journal of Infection
Bouzidi, Driouich, Klitting, Bernadin, Piorkowski et al., Generation and evaluation of protease inhibitor-resistant SARS-CoV-2 strains, Antiviral Research
Chang, Peng, Lee, Yang, Lin et al., Transporter modulation of molnupiravir and its metabolite β-D-N4-hydroxycytidine across the blood-brain barrier in a rat, Communications Medicine
Chatterjee, Bhattacharya, Dhama, Lee, Chakraborty, Molnupiravir's mechanism of action drives "error catastrophe" in SARS-CoV-2: A therapeutic strategy that leads to lethal mutagenesis of the virus, Mol Ther Nucleic Acids
Chen, Huang, Liu, Sun, Ji et al., Comparative characterization of SARS-CoV-2 variants of concern and mouse-adapted strains in mice, J Med Virol
Cooney, New chronic Covid study offers insight into which immunocompromised patients are most at risk, STAT
Cox, Lieber, Wolf, Karimi, Lieberman et al., Comparing molnupiravir and nirmatrelvir/ritonavir efficacy and the effects on SARS-CoV-2 transmission in animal models, Nat Commun
Cox, Wolf, Lieber, Sourimant, Lin et al., Oral prodrug of remdesivir parent GS-441524 is efficacious against SARS-CoV-2 in ferrets, Nat Commun
Cox, Wolf, Plemper, Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets, Nat Microbiol
Currey, Rabito, Maness, Blair, Rappaport et al., C57BL/6J Mice Are Not Suitable for Modeling Severe SARS-CoV-2 Beta and Gamma Variant Infection, Viruses
Da, Franco-Muñoz, Laiton-Donato, Usme-Ciro, Franco-Sierra et al., Molecular analysis of several in-house rRT-PCR protocols for SARS-CoV-2 detection in the context of genetic variability of the virus in Colombia, Infect Genet Evol
Davis, Mccorkell, Vogel, Topol, Long COVID: major findings, mechanisms and recommendations, Nature Reviews Microbiology
Dinnon, Leist, Schäfer, Edwards, Martinez et al., A mouse-adapted model of SARS-CoV-2 to test COVID-19 countermeasures, Nature
Dutta, COVID-19 waves: variant dynamics and control, Scientific Reports
Evans, Dube, Lu, Yates, Arnetorp et al., Impact of COVID-19 on immunocompromised populations during the Omicron era: insights from the observational population-based INFORM study, The Lancet Regional Health -Europe
Gusev, Sarapultsev, Solomatina, Chereshnev, SARS-CoV-2-Specific Immune Response and the Pathogenesis of COVID-19, Int J Mol Sci
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle et al., Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, New England Journal of Medicine
Handley, Ryan, Davies, Bewley, Carnell et al., SARS-CoV-2 Disease Severity in the Golden Syrian Hamster Model of Infection Is Related to the Volume of Intranasal Inoculum, Viruses
Heilmann, Costacurta, Moghadasi, Ye, Pavan et al., SARS-CoV-2 3CL(pro) mutations selected in a VSV-based system confer resistance to nirmatrelvir, ensitrelvir, and GC376, Sci Transl Med
Hu, Lewandowski, Tan, Zhang, Morgan et al., Naturally occurring mutations of SARS-CoV-2 main protease confer drug resistance to nirmatrelvir
Huang, Shuai, Qiao, Hou, Zeng et al., A new generation Mpro inhibitor with potent activity against SARS-CoV-2 Omicron variants, Signal Transduction and Targeted Therapy
Huchting, Targeting viral genome synthesis as broad-spectrum approach against RNA virus infections, Antivir Chem Chemother
Ia, Penrice-Randal, Goldswain, Rzeszutek, Pilgrim et al., Characterisation of SARS-CoV-2 genomic variation in response to molnupiravir treatment in the AGILE Phase IIa clinical trial, Nature Communications
Kar, Johnson, Vanderheiden, Elrod, Floyd et al., CD4+ and CD8+ T cells are required to prevent SARS-CoV-2 persistence in the nasal compartment, bioRxiv
Knight, Montgomery, Fletcher, Baxter, Mouse Models for the Study of SARS-CoV-2 Infection, Comp Med
Kuehl, Dearing, Werts, Cox, Irshad et al., Design and validation of an exposure system for efficient inter-animal SARS-CoV-2 airborne transmission in Syrian hamsters, Microbiol Spectr
Lahouati, Cazanave, Labadie, Gohier, Guirlé et al., Outcomes of targeted treatment in immunocompromised patients with asymptomatic or mild COVID-19: a retrospective study, Scientific Reports
Lang, What do we know about covid in immunocompromised people?, BMJ
Lieber, Aggarwal, Yoon, Cox, Kang et al., 4'-Fluorouridine mitigates lethal infection with pandemic human and highly pathogenic avian influenza viruses, PLoS Pathog
Lieber, Cox, Sourimant, Wolf, Juergens et al., SARS-CoV-2 VOC type and biological sex affect molnupiravir efficacy in severe COVID-19 dwarf hamster model, Nat Commun
Lieber, Kang, Aggarwal, Lieberman, Sobolik et al., Influenza A virus resistance to 4'fluorouridine coincides with viral attenuation in vitro and in vivo, PLoS Pathog
Lieber, Plemper, 4'-Fluorouridine Is a Broad-Spectrum Orally Available First-Line Antiviral That May Improve Pandemic Preparedness, DNA Cell Biol
Liu, Tsai, Unveiling COVID-19 treatment strategies for immunocompromised individuals: Therapeutic innovations and latest findings, Int J Rheum Dis
Meganck, Baric, Developing therapeutic approaches for twenty-firstcentury emerging infectious viral diseases, Nature Medicine
Moghadasi, Heilmann, Khalil, Nnabuife, Kearns et al., Transmissible SARS-CoV-2 variants with resistance to clinical protease inhibitors, bioRxiv
Nittas, Gao, West, Ballouz, Menges et al., Long COVID Through a Public Health Lens: An Umbrella Review, Public Health Rev
Painter, Holman, Bush, Almazedi, Malik et al., Human Safety, Tolerability, and Pharmacokinetics of Molnupiravir, a Novel Broad-Spectrum Oral Antiviral Agent with Activity Against SARS-CoV-2, Antimicrob Agents Chemother
Port, Morris, Riopelle, Yinda, Avanzato et al., Host and viral determinants of airborne transmission of SARS-CoV-2 in the Syrian hamster, Elife
Sanderson, Hisner, Donovan-Banfield, Hartman, Løchen et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature
Shahgolzari, Yavari, Arjeini, Miri, Darabi et al., Immunopathology and Immunopathogenesis of COVID-19, what we know and what we should learn, Gene Rep
Shoham, Batista, Amor, Ergonul, Hassanain et al., Vaccines and therapeutics for immunocompromised patients with COVID-19, eClinicalMedicine
Sia, Yan, Chin, Fung, Choy et al., Pathogenesis and transmission of SARS-CoV-2 in golden hamsters, Nature
Sourimant, Lieber, Aggarwal, Cox, Wolf et al., 4'-Fluorouridine is an oral antiviral that blocks respiratory syncytial virus and SARS-CoV-2 replication, Science
Standing, Buggiotti, Guerra-Assuncao, Woodall, Ellis et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications
Strizki, Gaspar, Howe, Hutchins, Mohri et al., Molnupiravir maintains antiviral activity against SARS-CoV-2 variants and exhibits a high barrier to the development of resistance, Antimicrob Agents Chemother
Sun, Liu, Huang, Xu, Hu et al., SARS-CoV-2 non-structural protein 6 triggers NLRP3-dependent pyroptosis by targeting ATP6AP1, Cell Death Differ
Swain, Lin, Wallentin, COVID-19 pandemic waves: Identification and interpretation of global data, Heliyon
Tan, Lam, Richard, Owen, Berchtold et al., Transmission of SARS-CoV-2 from humans to animals and potential host adaptation, Nat Commun
Toots, Yoon, Cox, Hart, Sticher et al., Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia, Sci. Transl. Med
Torbati, Krause, Ussher, The Immune Response to SARS-CoV-2 and Variants of Concern, Viruses
Trimpert, Vladimirova, Dietert, Abdelgawad, Kunec et al., The Roborovski Dwarf Hamster Is A Highly Susceptible Model for a Rapid and Fatal Course of SARS-CoV-2 Infection, Cell reports
Trøseid, Hentzien, Ader, Cardoso, Arribas et al., Immunocompromised patients have been neglected in COVID-19 trials: a call for action, Clin Microbiol Infect
Wilkinson, Richter, Casey, Osman, Mirza et al., Recurrent SARS-CoV-2 mutations in immunodeficient patients, Virus Evol
Wong, Lau, Au, Lau, Poon et al., Viral burden rebound in hospitalised patients with COVID-19 receiving oral antivirals in Hong Kong: a population-wide retrospective cohort study, The Lancet Infectious Diseases
Xie, Bowe, Al-Aly, Molnupiravir and risk of hospital admission or death in adults with covid-19: emulation of a randomized target trial using electronic health records, BMJ
Yang, Liu, Liu, Zhang, Wan et al., COVID-19: immunopathogenesis and Immunotherapeutics, Signal Transduction and Targeted Therapy
Zibat, Zhang, Dickmanns, Stegmann, Dobbelstein et al., N4hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience
DOI record:
{
"DOI": "10.1128/jvi.00905-24",
"ISSN": [
"0022-538X",
"1098-5514"
],
"URL": "http://dx.doi.org/10.1128/jvi.00905-24",
"abstract": "<jats:title>ABSTRACT</jats:title>\n <jats:sec>\n <jats:title/>\n <jats:p>\n Immunocompromised people are at high risk of prolonged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and progression to severe coronavirus disease 2019 (COVID-19). However, the efficacy of late-onset direct-acting antiviral (DAA) therapy with therapeutics in clinical use and experimental drugs to mitigate persistent viral replication is unclear. In this study, we employed an immunocompromised mouse model, which supports prolonged replication of SARS-CoV-2 to explore late-onset treatment options. Tandem immuno-depletion of CD4\n <jats:sup>+</jats:sup>\n and CD8\n <jats:sup>+</jats:sup>\n T cells in C57BL/6 mice followed by infection with SARS-CoV-2 variant of concern (VOC) beta B.1.351 resulted in prolonged infection with virus replication for 5 weeks after inoculation. Early-onset treatment with nirmatrelvir/ritonavir (paxlovid) or molnupiravir was only moderately efficacious, whereas the experimental therapeutic 4’-fluorouridine (4’-FlU, EIDD-2749) significantly reduced virus load in the upper and lower respiratory compartments 4 days postinfection (dpi). All antivirals significantly lowered virus burden in a 7-day treatment regimen initiated 14 dpi, but paxlovid-treated animals experienced rebound virus replication in the upper respiratory tract 7 days after treatment end. Viral RNA was detectable 28 dpi in paxlovid-treated animals, albeit not in the molnupiravir or 4’-FlU groups, when treatment was initiated 14 dpi and continued for 14 days. Low-level virus replication continued 35 dpi in animals receiving vehicle but had ceased in all treatment groups. These data indicate that late-onset DAA therapy significantly shortens the duration of persistent virus replication in an immunocompromised host, which may have implications for clinical use of antiviral therapeutics to alleviate the risk of progression to severe disease in highly vulnerable patients.\n </jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>IMPORTANCE</jats:title>\n <jats:p>\n Four years after the onset of the global coronavirus disease 2019 (COVID-19) pandemic, the immunocompromised are at greatest risk of developing life-threatening severe disease. However, specific treatment plans for this most vulnerable patient group have not yet been developed. Employing a CD4\n <jats:sup>+</jats:sup>\n and CD8\n <jats:sup>+</jats:sup>\n T cell-depleted immunocompromised mouse model of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, we explored therapeutic options of persistent infections with standard-of-care paxlovid, molnupiravir, and the experimental therapeutic 4’-fluorouridine (4’-FlU). Late-onset treatment initiated 14 days after infection was efficacious, but only 4’-FlU was rapidly sterilizing. No treatment-experienced viral variants with reduced susceptibility to the drugs emerged, albeit virus replication rebounded in animals of the paxlovid group after treatment end. This study supports the use of direct-acting antivirals (DAAs) for late-onset management of persistent SARS-CoV-2 infection in immunocompromised hosts. However, treatment courses likely require to be extended for maximal therapeutic benefit, calling for appropriately powered clinical trials to meet the specific needs of this patient group.\n </jats:p>\n </jats:sec>",
"alternative-id": [
"10.1128/jvi.00905-24"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2024-05-22"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2024-07-31"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2024-08-29"
}
],
"author": [
{
"affiliation": [
{
"name": "Center for Translational Antiviral Research, Georgia State University Institute for Biomedical Sciences, Atlanta, Georgia, USA"
}
],
"family": "Lieber",
"given": "Carolin M.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Center for Translational Antiviral Research, Georgia State University Institute for Biomedical Sciences, Atlanta, Georgia, USA"
}
],
"family": "Kang",
"given": "Hae-Ji",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Virology Division, Department of Laboratory Medicine and Pathology, University of Washington Medical Center, Seattle, Washington, USA"
}
],
"family": "Sobolik",
"given": "Elizabeth B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Emory Institute for Drug Development, Emory University, Atlanta, Georgia, USA"
}
],
"family": "Sticher",
"given": "Zachary M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Center for Translational Antiviral Research, Georgia State University Institute for Biomedical Sciences, Atlanta, Georgia, USA"
}
],
"family": "Ngo",
"given": "Vu L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Center for Translational Antiviral Research, Georgia State University Institute for Biomedical Sciences, Atlanta, Georgia, USA"
}
],
"family": "Gewirtz",
"given": "Andrew T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Emory Institute for Drug Development, Emory University, Atlanta, Georgia, USA"
}
],
"family": "Kolykhalov",
"given": "Alexander A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Emory Institute for Drug Development, Emory University, Atlanta, Georgia, USA"
}
],
"family": "Natchus",
"given": "Michael G.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7443-0527",
"affiliation": [
{
"name": "Virology Division, Department of Laboratory Medicine and Pathology, University of Washington Medical Center, Seattle, Washington, USA"
}
],
"authenticated-orcid": true,
"family": "Greninger",
"given": "Alexander L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Center for Childhood Infections and Vaccines of Children’s Healthcare of Atlanta, Department of Pediatrics, Emory University School of Medicine, Atlanta, Georgia, USA"
}
],
"family": "Suthar",
"given": "Mehul S.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2034-2107",
"affiliation": [
{
"name": "Center for Translational Antiviral Research, Georgia State University Institute for Biomedical Sciences, Atlanta, Georgia, USA"
}
],
"authenticated-orcid": true,
"family": "Plemper",
"given": "Richard K.",
"sequence": "additional"
}
],
"container-title": "Journal of Virology",
"container-title-short": "J Virol",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.asm.org"
]
},
"created": {
"date-parts": [
[
2024,
8,
29
]
],
"date-time": "2024-08-29T13:02:12Z",
"timestamp": 1724936532000
},
"deposited": {
"date-parts": [
[
2024,
9,
17
]
],
"date-time": "2024-09-17T17:05:08Z",
"timestamp": 1726592708000
},
"editor": [
{
"affiliation": [],
"family": "Schultz-Cherry",
"given": "Stacey",
"sequence": "additional"
}
],
"funder": [
{
"DOI": "10.13039/100000060",
"award": [
"AI171403,AI141222"
],
"doi-asserted-by": "crossref",
"id": [
{
"asserted-by": "crossref",
"id": "10.13039/100000060",
"id-type": "DOI"
}
],
"name": "HHS | NIH | National Institute of Allergy and Infectious Diseases"
}
],
"indexed": {
"date-parts": [
[
2024,
9,
18
]
],
"date-time": "2024-09-18T04:03:07Z",
"timestamp": 1726632187111
},
"is-referenced-by-count": 0,
"issue": "9",
"issued": {
"date-parts": [
[
2024,
9,
17
]
]
},
"journal-issue": {
"issue": "9",
"published-print": {
"date-parts": [
[
2024,
9,
17
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://doi.org/10.1128/ASMCopyrightv2",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
9,
17
]
],
"date-time": "2024-09-17T00:00:00Z",
"timestamp": 1726531200000
}
},
{
"URL": "https://journals.asm.org/non-commercial-tdm-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
9,
17
]
],
"date-time": "2024-09-17T00:00:00Z",
"timestamp": 1726531200000
}
}
],
"link": [
{
"URL": "https://journals.asm.org/doi/pdf/10.1128/jvi.00905-24",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journals.asm.org/doi/pdf/10.1128/jvi.00905-24",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "235",
"original-title": [],
"prefix": "10.1128",
"published": {
"date-parts": [
[
2024,
9,
17
]
]
},
"published-print": {
"date-parts": [
[
2024,
9,
17
]
]
},
"publisher": "American Society for Microbiology",
"reference": [
{
"DOI": "10.1016/j.heliyon.2024.e25090",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_3_2"
},
{
"DOI": "10.1038/s41598-022-13371-2",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_4_2"
},
{
"key": "e_1_3_5_5_2",
"unstructured": "NIH. 2024. Special considerations in people who are immunocompromised. Available from: https://www.covid19treatmentguidelines.nih.gov/special-populations/immunocompromised/"
},
{
"DOI": "10.1136/bmj.p1612",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_6_2"
},
{
"DOI": "10.1093/infdis/jiad181",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_7_2"
},
{
"key": "e_1_3_5_8_2",
"unstructured": "CDC. 2023. Long COVID and significant activity limitation among adults by age — United States. Available from: https://www.cdc.gov/mmwr/volumes/72/wr/mm7232a3.html"
},
{
"DOI": "10.3389/phrs.2022.1604501",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_9_2"
},
{
"DOI": "10.1038/s41579-022-00846-2",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_11_2"
},
{
"DOI": "10.1016/j.lanepe.2023.100747",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_13_2"
},
{
"DOI": "10.1126/scitranslmed.adk1599",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_14_2"
},
{
"DOI": "10.1001/jama.2021.7212",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_15_2"
},
{
"DOI": "10.1016/j.jinf.2021.01.022",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_16_2"
},
{
"DOI": "10.1016/j.cmi.2022.05.005",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_17_2"
},
{
"DOI": "10.1016/j.eclinm.2023.101965",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_18_2"
},
{
"DOI": "10.1038/s41598-023-42727-5",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_19_2"
},
{
"DOI": "10.1111/1756-185X.14900",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_20_2"
},
{
"DOI": "10.1038/s41564-020-00835-2",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_21_2"
},
{
"DOI": "10.1126/science.abj5508",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_22_2"
},
{
"DOI": "10.1038/s41467-021-26760-4",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_23_2"
},
{
"DOI": "10.1038/s41467-023-40556-8",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_24_2"
},
{
"DOI": "10.7554/eLife.87094.3",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_25_2"
},
{
"DOI": "10.1038/s41467-024-45348-2",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_26_2"
},
{
"DOI": "10.1038/s41467-022-32045-1",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_27_2"
},
{
"DOI": "10.3390/v16010122",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_28_2"
},
{
"DOI": "10.1128/spectrum.04717-22",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_29_2"
},
{
"DOI": "10.3390/v15030748",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_30_2"
},
{
"DOI": "10.1038/s41586-020-2342-5",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_31_2"
},
{
"DOI": "10.1016/j.celrep.2020.108488",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_32_2"
},
{
"DOI": "10.1002/jmv.27735",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_33_2"
},
{
"DOI": "10.30802/AALAS-CM-21-000031",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_34_2"
},
{
"DOI": "10.3390/v14050966",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_35_2"
},
{
"DOI": "10.1038/s41586-020-2708-8",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_36_2"
},
{
"DOI": "10.1038/s41423-020-00616-1",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_37_2"
},
{
"DOI": "10.1101/2024.01.23.576505",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_38_2"
},
{
"DOI": "10.1056/NEJMoa2118542",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_39_2"
},
{
"DOI": "10.1136/bmj-2022-072705",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_40_2"
},
{
"DOI": "10.1089/dna.2022.0312",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_42_2"
},
{
"DOI": "10.1371/journal.ppat.1011342",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_43_2"
},
{
"DOI": "10.1038/s41467-022-30698-6",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_44_2"
},
{
"DOI": "10.3390/v13101911",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_45_2"
},
{
"DOI": "10.1093/ve/veac050",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_46_2"
},
{
"DOI": "10.1038/s41418-021-00916-7",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_47_2"
},
{
"DOI": "10.1016/j.isci.2023.107786",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_48_2"
},
{
"DOI": "10.1038/s43856-023-00383-w",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_49_2"
},
{
"DOI": "10.1038/s41591-021-01282-0",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_50_2"
},
{
"DOI": "10.1177/2040206620976786",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_51_2"
},
{
"DOI": "10.3390/ijms23031716",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_52_2"
},
{
"DOI": "10.1016/j.genrep.2021.101417",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_53_2"
},
{
"DOI": "10.1038/s41392-020-00243-2",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_54_2"
},
{
"DOI": "10.1126/scitranslmed.aax5866",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_55_2"
},
{
"DOI": "10.1128/AAC.02428-20",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_56_2"
},
{
"DOI": "10.1016/j.omtn.2023.06.006",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_57_2"
},
{
"DOI": "10.1038/s41467-024-45641-0",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_58_2"
},
{
"DOI": "10.1038/s41586-023-06649-6",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_59_2"
},
{
"DOI": "10.1038/s41392-023-01392-w",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_60_2"
},
{
"DOI": "10.1016/S1473-3099(22)00873-8",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_61_2"
},
{
"DOI": "10.1016/j.antiviral.2024.105814",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_62_2"
},
{
"DOI": "10.1126/scitranslmed.abq7360",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_63_2"
},
{
"DOI": "10.1101/2022.08.07.503099",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_64_2",
"unstructured": "Moghadasi SA Heilmann E Khalil AM Nnabuife C Kearns FL Ye C Moraes SN Costacurta F Esler MA Aihara H von Laer D Martinez-Sobrido L Palzkill T Amaro RE Harris RS. 2022. Transmissible SARS-CoV-2 variants with resistance to clinical protease inhibitors. bioRxiv:2022.08.07.503099. doi:10.1101/2022.08.07.503099"
},
{
"DOI": "10.1101/2022.06.28.497978",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_65_2"
},
{
"DOI": "10.1128/aac.00953-23",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_66_2"
},
{
"DOI": "10.1038/s41467-022-34839-9",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_67_2"
},
{
"DOI": "10.1371/journal.ppat.1011993",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_68_2"
},
{
"DOI": "10.1128/AAC.00766-18",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_69_2"
},
{
"DOI": "10.1126/science.abl4784",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_70_2"
},
{
"DOI": "10.1016/j.meegid.2020.104390",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_71_2"
}
],
"reference-count": 66,
"references-count": 66,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.asm.org/doi/10.1128/jvi.00905-24"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficacy of late-onset antiviral treatment in immunocompromised hosts with persistent SARS-CoV-2 infection",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1128/asmj-crossmark-policy-page",
"volume": "98"
}
lieber2