Olgotrelvir as a Single-Agent Treatment of Nonhospitalized Patients with Covid-19
et al., NEJM Evidence, doi:10.1056/EVIDoa2400026, MPR-COV-301CN, NCT05716425, May 2024
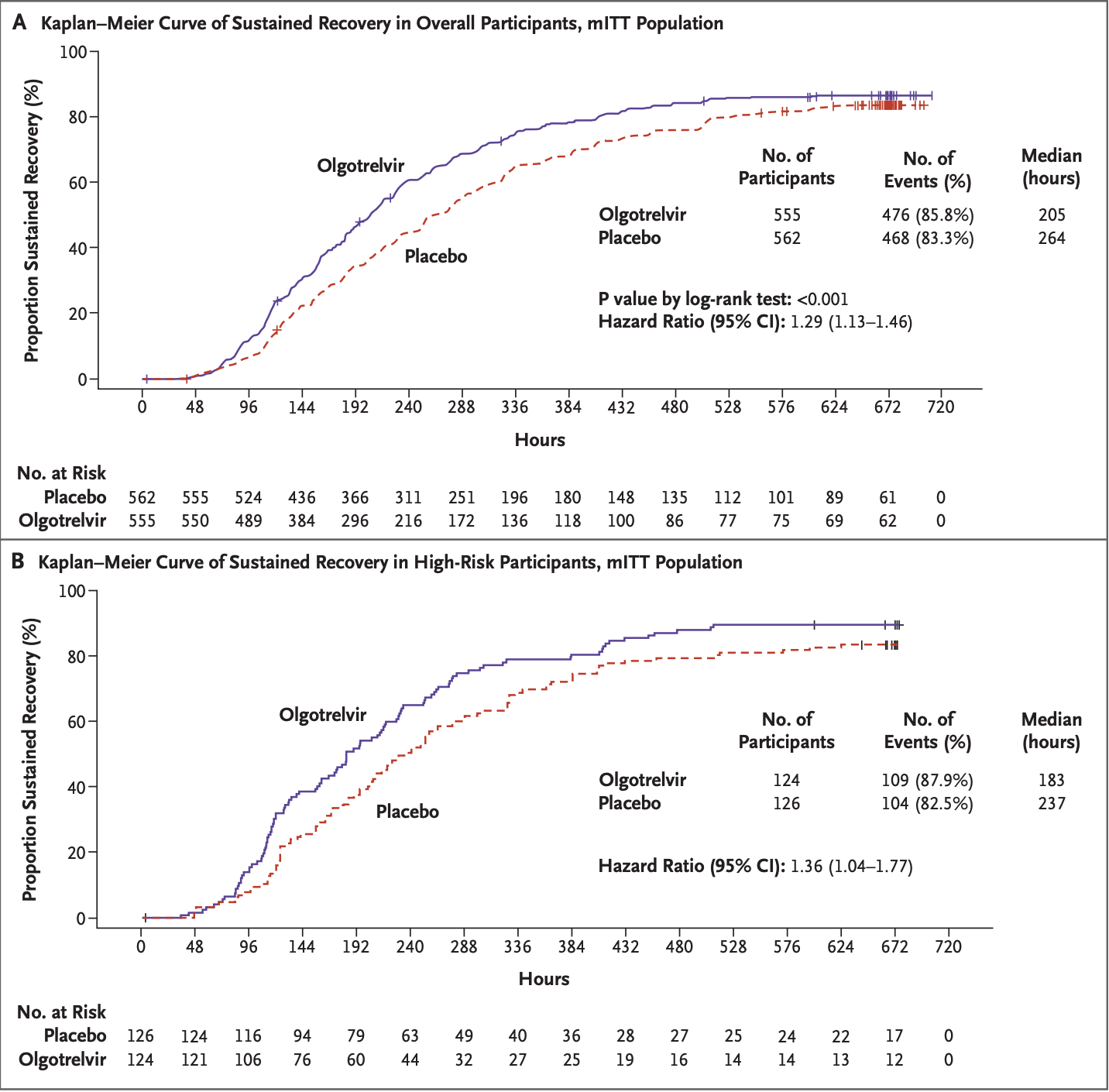
RCT 1,212 outpatients with mild to moderate COVID-19 showing faster recovery with olgotrelvir.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
|
risk of oxygen therapy, 66.6% lower, RR 0.33, p = 1.00, treatment 0 of 604 (0.0%), control 1 of 608 (0.2%), NNT 608, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of hospitalization, 66.6% lower, RR 0.33, p = 1.00, treatment 0 of 604 (0.0%), control 1 of 608 (0.2%), NNT 608, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of no recovery, 22.5% lower, HR 0.78, p < 0.001, treatment 604, control 608, inverted to make HR<1 favor treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Jiang et al., 28 May 2024, Double Blind Randomized Controlled Trial, placebo-controlled, China, peer-reviewed, median age 33.0, 44 authors, study period February 2023 - June 2023, average treatment delay 2.0 days, trial NCT05716425 (history) (MPR-COV-301CN).
Contact: xxu@aceatherapeutics.com, 13911900791@163.com, luhongzhou@szsy.sustech.edu.cn.
Olgotrelvir as a Single-Agent Treatment of Nonhospitalized Patients with Covid-19
NEJM Evidence, doi:10.1056/evidoa2400026
BACKGROUND Olgotrelvir is an oral antiviral with dual mechanisms of action targeting severe acute respiratory syndrome coronavirus 2 main protease (i.e., M pro ) and human cathepsin L. It has potential to serve as a single-agent treatment of coronavirus disease 2019 (Covid-19). METHODS We conducted a phase 3, double-blind, randomized, placebo-controlled trial to evaluate the efficacy and safety of olgotrelvir in 1212 nonhospitalized adult participants with mild to moderate Covid-19, irrespective of risk factors, who were randomly assigned to receive orally either 600 mg of olgotrelvir or placebo twice daily for 5 days. The primary and key secondary end points were time to sustained recovery of a panel of 11 Covid-19-related symptoms and the viral ribonucleic acid (RNA) load. The safety end point was incidence of treatment-emergent adverse events.
RESULTS The baseline characteristics of 1212 participants were similar in the two groups. In the modified intention-to-treat population (567 patients in the placebo group and 558 in the olgotrelvir group), the median time to symptom recovery was 205 hours in the olgotrelvir group versus 264 hours in the placebo group (hazard ratio, 1.29; 95% confidence interval [CI], 1.13 to 1.46; P<0.001). The least squares mean (95% CI) changes of viral RNA load from baseline were -2.20 (-2.59 to -1.81) log 10 copies/ml in olgotrelvirtreated participants and -1.40 (-1.79 to -1.01) in participants receiving placebo at day 4. Skin rash (3.3%) and nausea (1.5%) were more frequent in the olgotrelvir group than in the placebo group; there were no treatment-related serious adverse events, and no deaths were reported.
Author Affiliations
References
Andrews, Stowe, Kirsebom, Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant, N Engl J Med, doi:10.1056/NEJMoa2119451
Beigel, Tomashek, Dodd, Remdesivir for the treatment of Covid-19 -final report, N Engl J Med, doi:10.1056/NEJMoa2007764
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Hammond, Fountaine, Yunis, Nirmatrelvir for vaccinated or unvaccinated adult outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2309003
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Kissler, Fauver, Mack, Viral dynamics of acute SARS-CoV-2 infection and applications to diagnostic and public health strategies, PLoS Biol, doi:10.1371/journal.pbio.3001333
Liu, Luo, Libby, Shi, Cathepsin L-selective inhibitors: a potentially promising treatment for COVID-19 patients, Pharmacol Ther, doi:10.1016/j.pharmthera.2020.107587
Mao, Shaabani, Zhang, An oral antiviral olgotrelvir with dual inhibitory activities of SARS CoV-2 Mpro and cathepsin L as a standalone intervention candidate for COVID-19, Med
Markov, Ghafari, Beer, The evolution of SARS-CoV-2, Nat Rev Microbiol, doi:10.1038/s41579-023-00878-2
Meng, Abdullahi, Ferreira, Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity, Nature, doi:10.1038/s41586-022-04474-x
Mishra, Mindermann, Sharma, Changing composition of SARS-CoV-2 lineages and rise of Delta variant in England, EClinical-Medicine, doi:10.1016/j.eclinm.2021.101064
Puhach, Meyer, Eckerle, SARS-CoV-2 viral load and shedding kinetics, Nat Rev Microbiol, doi:10.1038/s41579-022-00822-w
Willett, Grove, Maclean, SARS-CoV-2 Omicron is an immune escape variant with an altered cell entry pathway [published correction appears in, Nat Microbiol, doi:10.1038/s41564-022-01143-7
Wu, Chen, Cai, Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China, JAMA Intern Med, doi:10.1001/jamainternmed.2020.0994
Zhu, Zhang, Wang, A novel coronavirus from patients with pneumonia in China, 2019, N Engl J Med, doi:10.1056/NEJMoa2001017
DOI record:
{
"DOI": "10.1056/evidoa2400026",
"ISSN": [
"2766-5526"
],
"URL": "http://dx.doi.org/10.1056/EVIDoa2400026",
"alternative-id": [
"10.1056/EVIDoa2400026"
],
"author": [
{
"affiliation": [
{
"name": "Beijing Ditan Hospital Capital Medical University, China"
}
],
"family": "Jiang",
"given": "Rongmeng",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Beijing Ditan Hospital Capital Medical University, China"
}
],
"family": "Han",
"given": "Bing",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "ACEA Pharmaceutical Co., Ltd., Hangzhou, Zhejiang, China"
}
],
"family": "Xu",
"given": "Wanhong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "ACEA Pharmaceutical Co., Ltd., Hangzhou, Zhejiang, China"
}
],
"family": "Zhang",
"given": "Xiaoying",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "People’s Hospital of Quzhou City, Quzhou, Zhejiang, China"
}
],
"family": "Peng",
"given": "Chunxian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Nanyang Central Hospital, Nanyang, Henan, China"
}
],
"family": "Dang",
"given": "Qiang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "People’s Hospital of Chongqing Banan District, Chongqing, China"
}
],
"family": "Sun",
"given": "Wei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hainan Third People’s Hospital, Sanya, Hainan, China"
}
],
"family": "Lin",
"given": "Ling",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Shenzhen Third People’s Hospital, SUSTech, Shenzhen, China"
}
],
"family": "Lin",
"given": "Yuanlong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ningbo No. 2 Hospital, Ningbo, Zhejiang, China"
}
],
"family": "Fan",
"given": "Lingyan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Taizhou Hospital of Zhejiang Province, Taizhou, Zhejiang, China"
}
],
"family": "Lv",
"given": "Dongqing",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Jinan Central Hospital, Jinan, Shandong, China"
}
],
"family": "Shao",
"given": "Lei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The Second People’s Hospital of Changde, Changde, Hunan, China"
}
],
"family": "Chen",
"given": "Ying",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The First Affiliated Hospital Zhejiang University School of Medicine, Hangzhou, Zhejiang, China"
}
],
"family": "Qiu",
"given": "Yunqing",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The Second Affiliated Hospital of Xinjiang Medical University, Urumqi, Xinjiang, China"
}
],
"family": "Han",
"given": "Limei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Jining No. 1 People’s Hospital, Jining, China"
}
],
"family": "Kong",
"given": "Weixiang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The Sixth People’s Hospital of Zhengzhou, Henan, China"
}
],
"family": "Li",
"given": "Guangming",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The Fourth Affiliated Hospital Zhejiang University School of Medicine, Yiwu, Zhejiang, China"
}
],
"family": "Wang",
"given": "Kai",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Nangfang Hospital Southern Medical University, Guangzhou, Guangdong, China"
}
],
"family": "Peng",
"given": "Jie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, China"
}
],
"family": "Lin",
"given": "Bingliang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Huzhou Central Hospital, Huzhou, Zhejiang, China"
}
],
"family": "Tong",
"given": "Zhaowei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The First Teaching Hospital of Xinjiang Medical University, Urumqi, Xinjiang, China"
}
],
"family": "Lu",
"given": "Xiaobo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Xi’an Gaoxin Hospital, Xi’an, Shanxi, China"
}
],
"family": "Wang",
"given": "Lifeng",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Linyi People’s Hospital, Linyi, Shandong, China"
}
],
"family": "Gao",
"given": "Feng",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Guigang City People’s Hospital, Guiyang, Guangxi, China"
}
],
"family": "Feng",
"given": "Jiemei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The Second Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China"
}
],
"family": "Li",
"given": "Yongxia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Linfen Central Hospital, Linfeng, Shanxi, China"
}
],
"family": "Ma",
"given": "Xiaojun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Beijing Luhe Hospital Affiliated Capital Medical University, Beijing, China"
}
],
"family": "Wang",
"given": "Jinxiang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "ACEA Pharmaceutical Co., Ltd., Hangzhou, Zhejiang, China"
}
],
"family": "Wang",
"given": "Shanbo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "ACEA Pharmaceutical Co., Ltd., Hangzhou, Zhejiang, China"
}
],
"family": "Shen",
"given": "Wei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "ACEA Pharmaceutical Co., Ltd., Hangzhou, Zhejiang, China"
}
],
"family": "Wang",
"given": "Chao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "ACEA Pharmaceutical Co., Ltd., Hangzhou, Zhejiang, China"
}
],
"family": "Yan",
"given": "Kuan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "ACEA Pharmaceutical Co., Ltd., Hangzhou, Zhejiang, China"
}
],
"family": "Lin",
"given": "Zhenhao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "ACEA Therapeutics, Inc., San Diego, CA"
}
],
"family": "Jin",
"given": "Can",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "ACEA Therapeutics, Inc., San Diego, CA"
}
],
"family": "Mao",
"given": "Long",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "ACEA Therapeutics, Inc., San Diego, CA"
}
],
"family": "Liu",
"given": "Jia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "ACEA Therapeutics, Inc., San Diego, CA"
}
],
"family": "Kushnareva",
"given": "Yulia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "ACEA Therapeutics, Inc., San Diego, CA"
}
],
"family": "Kotoi",
"given": "Olivia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "ACEA Therapeutics, Inc., San Diego, CA"
}
],
"family": "Zhu",
"given": "Zhenhong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Sorrento Therapeutics, Inc., San Diego, CA"
}
],
"family": "Royal",
"given": "Mike",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Sorrento Therapeutics, Inc., San Diego, CA"
}
],
"family": "Brunswick",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Sorrento Therapeutics, Inc., San Diego, CA"
}
],
"family": "Ji",
"given": "Henry",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "ACEA Therapeutics, Inc., San Diego, CA"
}
],
"family": "Xu",
"given": "Xiao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Shenzhen Third People’s Hospital, SUSTech, Shenzhen, China"
}
],
"family": "Lu",
"given": "Hongzhou",
"sequence": "additional"
}
],
"container-title": "NEJM Evidence",
"container-title-short": "NEJM Evidence",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
5,
28
]
],
"date-time": "2024-05-28T13:30:31Z",
"timestamp": 1716903031000
},
"deposited": {
"date-parts": [
[
2025,
1,
30
]
],
"date-time": "2025-01-30T20:12:18Z",
"timestamp": 1738267938000
},
"indexed": {
"date-parts": [
[
2025,
8,
22
]
],
"date-time": "2025-08-22T05:15:21Z",
"timestamp": 1755839721974,
"version": "3.35.0"
},
"is-referenced-by-count": 5,
"issue": "6",
"issued": {
"date-parts": [
[
2024,
5,
28
]
]
},
"journal-issue": {
"issue": "6",
"published-print": {
"date-parts": [
[
2024,
5,
28
]
]
}
},
"language": "en",
"member": "150",
"original-title": [],
"prefix": "10.1056",
"published": {
"date-parts": [
[
2024,
5,
28
]
]
},
"published-print": {
"date-parts": [
[
2024,
5,
28
]
]
},
"publisher": "Massachusetts Medical Society",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_2_2"
},
{
"key": "e_1_3_5_3_2",
"unstructured": "World Health Organization. Coronavirus disease 2019 (COVID-19). Situation report — 51. March 11 2020 (https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10)."
},
{
"DOI": "10.1016/j.eclinm.2021.101064",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_4_2"
},
{
"DOI": "10.1056/NEJMoa2119451",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_5_2"
},
{
"DOI": "10.1038/s41579-023-00878-2",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_6_2"
},
{
"DOI": "10.1001/jamainternmed.2020.0994",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_7_2"
},
{
"DOI": "10.1016/j.medj.2023.12.004",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_8_2"
},
{
"DOI": "10.1016/j.pharmthera.2020.107587",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_9_2"
},
{
"DOI": "10.1038/s41564-022-01143-7",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_10_2"
},
{
"DOI": "10.1038/s41586-022-04474-x",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_11_2"
},
{
"DOI": "10.1371/journal.pbio.3001333",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_12_2"
},
{
"DOI": "10.1038/s41579-022-00822-w",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_13_2"
},
{
"DOI": "10.1056/NEJMoa2118542",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_14_2"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_15_2"
},
{
"DOI": "10.1056/NEJMoa2116044",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_16_2"
},
{
"DOI": "10.1056/NEJMoa2309003",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_17_2"
}
],
"reference-count": 16,
"references-count": 16,
"relation": {},
"resource": {
"primary": {
"URL": "https://evidence.nejm.org/doi/10.1056/EVIDoa2400026"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Olgotrelvir as a Single-Agent Treatment of Nonhospitalized Patients with Covid-19",
"type": "journal-article",
"volume": "3"
}