Nirmatrelvir for Vaccinated or Unvaccinated Adult Outpatients with Covid-19
et al., New England Journal of Medicine, doi:10.1056/NEJMoa2309003, EPIC-SR, NCT05011513, Apr 2024
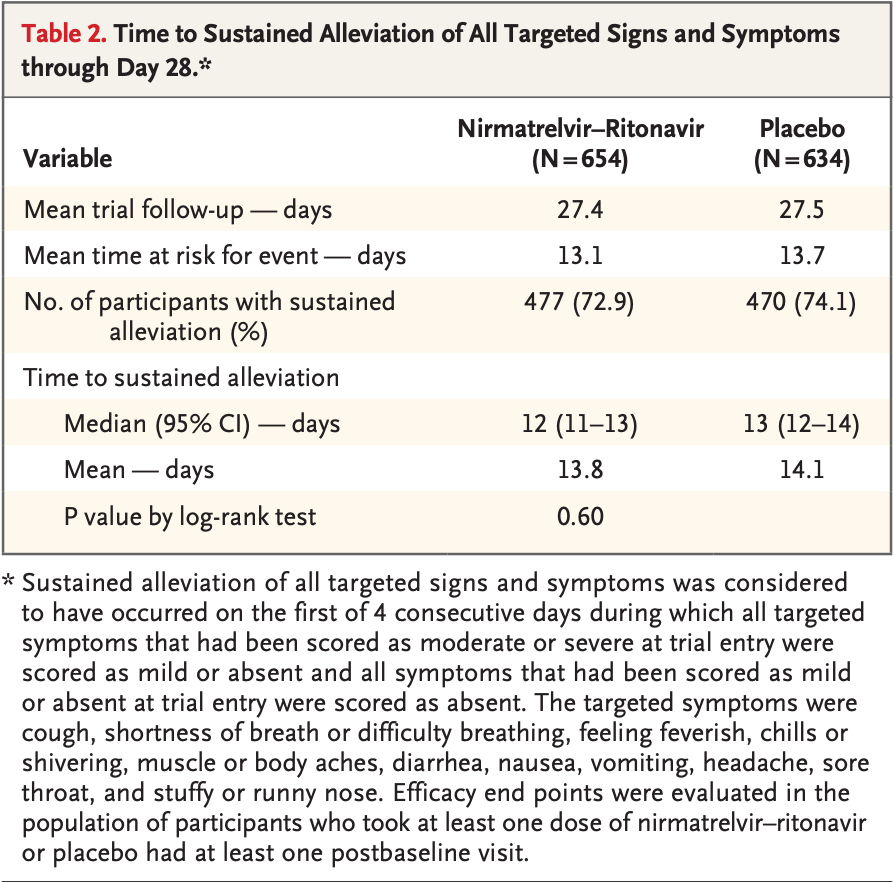
Delayed publication for EPIC-SR showing no significant difference in time to sustained alleviation. Selected results were first made available in December 20211.
Hammond et al., 4 Apr 2024, peer-reviewed, 13 authors, trial NCT05011513 (history) (EPIC-SR).
DOI record:
{
"DOI": "10.1056/nejmoa2309003",
"ISSN": [
"0028-4793",
"1533-4406"
],
"URL": "http://dx.doi.org/10.1056/NEJMoa2309003",
"alternative-id": [
"10.1056/NEJMoa2309003"
],
"author": [
{
"affiliation": [
{
"name": "From Global Product Development, Pfizer, Collegeville, PA (J.H.); Global Product Development, Pfizer, Groton, CT (R.J.F.); Global Product Development, Pfizer, Lake Mary (C.Y.), and Global Product Development, Pfizer, Tampa (J.M.R.) — both in Florida; Global Product Development, Pfizer, Lexington, KY (D.F.); Global Product Development, Pfizer, New York (M.A., W.B., R.P.); Global Product Development, Pfizer, Lake Forest, IL (W.W.); Early Clinical Development, Pfizer, Cambridge, MA (M.L.B.); Pfizer,..."
}
],
"family": "Hammond",
"given": "Jennifer",
"sequence": "first"
},
{
"affiliation": [
{
"name": "From Global Product Development, Pfizer, Collegeville, PA (J.H.); Global Product Development, Pfizer, Groton, CT (R.J.F.); Global Product Development, Pfizer, Lake Mary (C.Y.), and Global Product Development, Pfizer, Tampa (J.M.R.) — both in Florida; Global Product Development, Pfizer, Lexington, KY (D.F.); Global Product Development, Pfizer, New York (M.A., W.B., R.P.); Global Product Development, Pfizer, Lake Forest, IL (W.W.); Early Clinical Development, Pfizer, Cambridge, MA (M.L.B.); Pfizer,..."
}
],
"family": "Fountaine",
"given": "Robert J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Global Product Development, Pfizer, Collegeville, PA (J.H.); Global Product Development, Pfizer, Groton, CT (R.J.F.); Global Product Development, Pfizer, Lake Mary (C.Y.), and Global Product Development, Pfizer, Tampa (J.M.R.) — both in Florida; Global Product Development, Pfizer, Lexington, KY (D.F.); Global Product Development, Pfizer, New York (M.A., W.B., R.P.); Global Product Development, Pfizer, Lake Forest, IL (W.W.); Early Clinical Development, Pfizer, Cambridge, MA (M.L.B.); Pfizer,..."
}
],
"family": "Yunis",
"given": "Carla",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Global Product Development, Pfizer, Collegeville, PA (J.H.); Global Product Development, Pfizer, Groton, CT (R.J.F.); Global Product Development, Pfizer, Lake Mary (C.Y.), and Global Product Development, Pfizer, Tampa (J.M.R.) — both in Florida; Global Product Development, Pfizer, Lexington, KY (D.F.); Global Product Development, Pfizer, New York (M.A., W.B., R.P.); Global Product Development, Pfizer, Lake Forest, IL (W.W.); Early Clinical Development, Pfizer, Cambridge, MA (M.L.B.); Pfizer,..."
}
],
"family": "Fleishaker",
"given": "Dona",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Global Product Development, Pfizer, Collegeville, PA (J.H.); Global Product Development, Pfizer, Groton, CT (R.J.F.); Global Product Development, Pfizer, Lake Mary (C.Y.), and Global Product Development, Pfizer, Tampa (J.M.R.) — both in Florida; Global Product Development, Pfizer, Lexington, KY (D.F.); Global Product Development, Pfizer, New York (M.A., W.B., R.P.); Global Product Development, Pfizer, Lake Forest, IL (W.W.); Early Clinical Development, Pfizer, Cambridge, MA (M.L.B.); Pfizer,..."
}
],
"family": "Almas",
"given": "Mary",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Global Product Development, Pfizer, Collegeville, PA (J.H.); Global Product Development, Pfizer, Groton, CT (R.J.F.); Global Product Development, Pfizer, Lake Mary (C.Y.), and Global Product Development, Pfizer, Tampa (J.M.R.) — both in Florida; Global Product Development, Pfizer, Lexington, KY (D.F.); Global Product Development, Pfizer, New York (M.A., W.B., R.P.); Global Product Development, Pfizer, Lake Forest, IL (W.W.); Early Clinical Development, Pfizer, Cambridge, MA (M.L.B.); Pfizer,..."
}
],
"family": "Bao",
"given": "Weihang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Global Product Development, Pfizer, Collegeville, PA (J.H.); Global Product Development, Pfizer, Groton, CT (R.J.F.); Global Product Development, Pfizer, Lake Mary (C.Y.), and Global Product Development, Pfizer, Tampa (J.M.R.) — both in Florida; Global Product Development, Pfizer, Lexington, KY (D.F.); Global Product Development, Pfizer, New York (M.A., W.B., R.P.); Global Product Development, Pfizer, Lake Forest, IL (W.W.); Early Clinical Development, Pfizer, Cambridge, MA (M.L.B.); Pfizer,..."
}
],
"family": "Wisemandle",
"given": "Wayne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Global Product Development, Pfizer, Collegeville, PA (J.H.); Global Product Development, Pfizer, Groton, CT (R.J.F.); Global Product Development, Pfizer, Lake Mary (C.Y.), and Global Product Development, Pfizer, Tampa (J.M.R.) — both in Florida; Global Product Development, Pfizer, Lexington, KY (D.F.); Global Product Development, Pfizer, New York (M.A., W.B., R.P.); Global Product Development, Pfizer, Lake Forest, IL (W.W.); Early Clinical Development, Pfizer, Cambridge, MA (M.L.B.); Pfizer,..."
}
],
"family": "Baniecki",
"given": "Mary Lynn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Global Product Development, Pfizer, Collegeville, PA (J.H.); Global Product Development, Pfizer, Groton, CT (R.J.F.); Global Product Development, Pfizer, Lake Mary (C.Y.), and Global Product Development, Pfizer, Tampa (J.M.R.) — both in Florida; Global Product Development, Pfizer, Lexington, KY (D.F.); Global Product Development, Pfizer, New York (M.A., W.B., R.P.); Global Product Development, Pfizer, Lake Forest, IL (W.W.); Early Clinical Development, Pfizer, Cambridge, MA (M.L.B.); Pfizer,..."
}
],
"family": "Hendrick",
"given": "Victoria M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Global Product Development, Pfizer, Collegeville, PA (J.H.); Global Product Development, Pfizer, Groton, CT (R.J.F.); Global Product Development, Pfizer, Lake Mary (C.Y.), and Global Product Development, Pfizer, Tampa (J.M.R.) — both in Florida; Global Product Development, Pfizer, Lexington, KY (D.F.); Global Product Development, Pfizer, New York (M.A., W.B., R.P.); Global Product Development, Pfizer, Lake Forest, IL (W.W.); Early Clinical Development, Pfizer, Cambridge, MA (M.L.B.); Pfizer,..."
}
],
"family": "Kalfov",
"given": "Veselin",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9630-7792",
"affiliation": [
{
"name": "From Global Product Development, Pfizer, Collegeville, PA (J.H.); Global Product Development, Pfizer, Groton, CT (R.J.F.); Global Product Development, Pfizer, Lake Mary (C.Y.), and Global Product Development, Pfizer, Tampa (J.M.R.) — both in Florida; Global Product Development, Pfizer, Lexington, KY (D.F.); Global Product Development, Pfizer, New York (M.A., W.B., R.P.); Global Product Development, Pfizer, Lake Forest, IL (W.W.); Early Clinical Development, Pfizer, Cambridge, MA (M.L.B.); Pfizer,..."
}
],
"authenticated-orcid": false,
"family": "Simón-Campos",
"given": "J. Abraham",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Global Product Development, Pfizer, Collegeville, PA (J.H.); Global Product Development, Pfizer, Groton, CT (R.J.F.); Global Product Development, Pfizer, Lake Mary (C.Y.), and Global Product Development, Pfizer, Tampa (J.M.R.) — both in Florida; Global Product Development, Pfizer, Lexington, KY (D.F.); Global Product Development, Pfizer, New York (M.A., W.B., R.P.); Global Product Development, Pfizer, Lake Forest, IL (W.W.); Early Clinical Development, Pfizer, Cambridge, MA (M.L.B.); Pfizer,..."
}
],
"family": "Pypstra",
"given": "Rienk",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Global Product Development, Pfizer, Collegeville, PA (J.H.); Global Product Development, Pfizer, Groton, CT (R.J.F.); Global Product Development, Pfizer, Lake Mary (C.Y.), and Global Product Development, Pfizer, Tampa (J.M.R.) — both in Florida; Global Product Development, Pfizer, Lexington, KY (D.F.); Global Product Development, Pfizer, New York (M.A., W.B., R.P.); Global Product Development, Pfizer, Lake Forest, IL (W.W.); Early Clinical Development, Pfizer, Cambridge, MA (M.L.B.); Pfizer,..."
}
],
"family": "Rusnak",
"given": "James M.",
"sequence": "additional"
}
],
"container-title": "New England Journal of Medicine",
"container-title-short": "N Engl J Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
4,
3
]
],
"date-time": "2024-04-03T21:00:29Z",
"timestamp": 1712178029000
},
"deposited": {
"date-parts": [
[
2024,
4,
3
]
],
"date-time": "2024-04-03T21:01:17Z",
"timestamp": 1712178077000
},
"funder": [
{
"DOI": "10.13039/100004319",
"award": [
"N/A"
],
"doi-asserted-by": "publisher",
"name": "Pfizer"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
4
]
],
"date-time": "2024-04-04T01:11:38Z",
"timestamp": 1712193098768
},
"is-referenced-by-count": 1,
"issue": "13",
"issued": {
"date-parts": [
[
2024,
4,
4
]
]
},
"journal-issue": {
"issue": "13",
"published-print": {
"date-parts": [
[
2024,
4,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://www.nejmgroup.org/legal/terms-of-use.htm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
4,
4
]
],
"date-time": "2024-04-04T00:00:00Z",
"timestamp": 1712188800000
}
}
],
"link": [
{
"URL": "http://www.nejm.org/doi/pdf/10.1056/NEJMoa2309003",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "150",
"original-title": [],
"page": "1186-1195",
"prefix": "10.1056",
"published": {
"date-parts": [
[
2024,
4,
4
]
]
},
"published-print": {
"date-parts": [
[
2024,
4,
4
]
]
},
"publisher": "Massachusetts Medical Society",
"reference": [
{
"DOI": "10.1002/jmv.26326",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_2_2"
},
{
"DOI": "10.1001/jama.2020.2648",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_3_2"
},
{
"DOI": "10.1093/cid/ciaa1012",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_4_2"
},
{
"DOI": "10.1136/bmj.m1985",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_5_2"
},
{
"DOI": "10.1038/s41598-021-88130-w",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_6_2"
},
{
"DOI": "10.1111/febs.12936",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_7_2"
},
{
"DOI": "10.1126/science.abl4784",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_8_2"
},
{
"key": "e_1_3_5_9_2",
"unstructured": "Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization for Paxlovid. 2024 (https://labeling.pfizer.com/ShowLabeling.aspx?id=16474)."
},
{
"DOI": "10.1056/NEJMoa2118542",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_10_2"
},
{
"key": "e_1_3_5_11_2",
"unstructured": "Food and Drug Administration. FDA briefing document. Antimicrobial Drugs Advisory Committee meeting March 16 2023 (https://www.fda.gov/media/166197/download)."
},
{
"key": "e_1_3_5_12_2",
"unstructured": "Food and Drug Administration. Assessing COVID-19-related symptoms in outpatient adult and adolescent subjects in clinical trials of drugs and biological products for COVID-19 prevention or treatment. Guidance for industry. February 2024 (https://www.fda.gov/regulatory-information/search-fda-guidance-documents/assessing-covid-19-related-symptoms-outpatient-adult-and-adolescent-subjects-clinical-trials-drugs)."
},
{
"DOI": "10.1101/2022.10.02.22280623",
"doi-asserted-by": "crossref",
"key": "e_1_3_5_13_2",
"unstructured": "Lewnard JA McLaughlin JM Malden D et al. Effectiveness of nirmatrelvir-ritonavir against hospital admission or death: a cohort study in a large US healthcare system. January 10 2023 (https://www.medrxiv.org/content/10.1101/2022.10.02.22280623v2). preprint."
},
{
"DOI": "10.1016/S0140-6736(22)01586-0",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_14_2"
},
{
"DOI": "10.7326/M22-3565",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_15_2"
},
{
"DOI": "10.1503/cmaj.221608",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_16_2"
},
{
"DOI": "10.1056/NEJMoa2204919",
"doi-asserted-by": "publisher",
"key": "e_1_3_5_17_2"
}
],
"reference-count": 16,
"references-count": 16,
"relation": {},
"resource": {
"primary": {
"URL": "http://www.nejm.org/doi/10.1056/NEJMoa2309003"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Nirmatrelvir for Vaccinated or Unvaccinated Adult Outpatients with Covid-19",
"type": "journal-article",
"volume": "390"
}