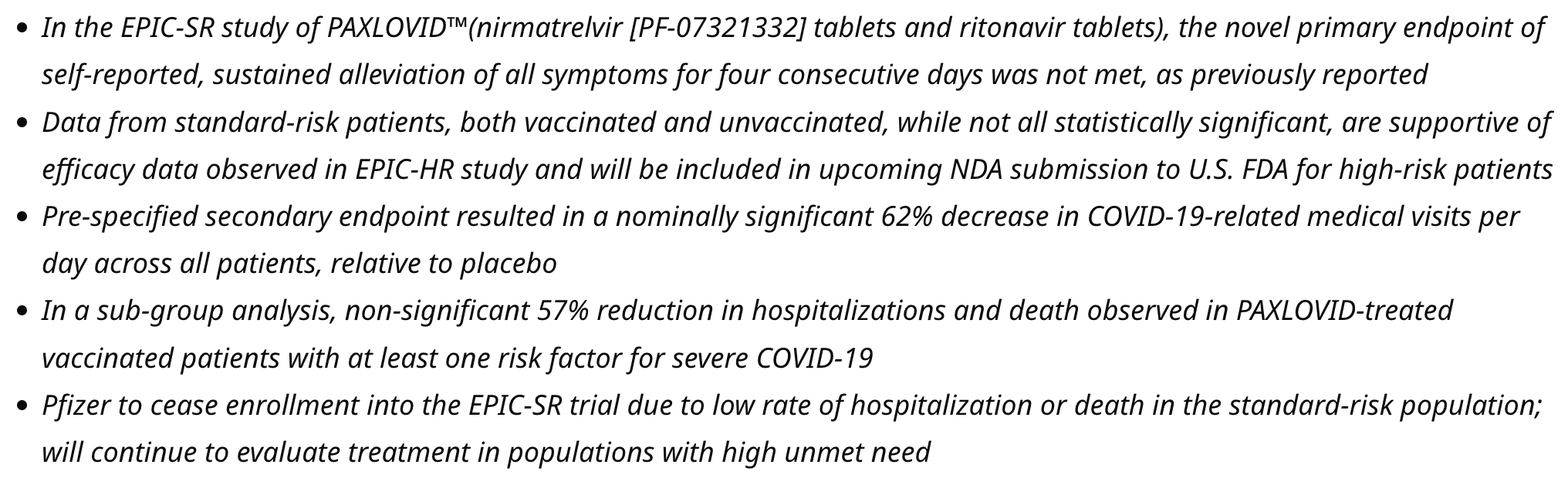
Evaluation of Protease Inhibition for COVID-19 in Standard-Risk Patients (EPIC-SR)
, NCT05011513, EPIC-SR, NCT05011513, Jun 2022
Results for the terminated and unpublished (until April 2024) EPIC-SR trial.
|
risk of death, 67.0% lower, RR 0.33, p = 0.49, treatment 0 of 654 (0.0%), control 1 of 634 (0.2%), NNT 634, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 28.
|
|
risk of hospitalization, 51.5% lower, RR 0.48, p = 0.20, treatment 5 of 654 (0.8%), control 10 of 634 (1.6%), NNT 123, day 28.
|
|
risk of progression, 3.8% lower, RR 0.96, p = 0.21, treatment 494 of 654 (75.5%), control 498 of 634 (78.5%), NNT 33, day 28.
|
|
relative time to sustained alleviation, 7.7% lower, relative time 0.92, p = 0.16, treatment mean 12.0 (±13.0) n=654, control mean 13.0 (±12.8) n=634.
|
|
relative change in viral load, 3.9% better, RR 0.96, p = 0.04, treatment mean 5.46 (±1.84) n=654, control mean 5.25 (±1.89) n=634, day 14.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Pfizer et al., 14 Jun 2022, Double Blind Randomized Controlled Trial, multiple countries, preprint, 1 author, study period 25 August, 2021 - 25 July, 2022, trial NCT05011513 (history) (EPIC-SR).