Findings from a discontinued clinical trial of favipiravir in high-risk patients with early-onset COVID-19
et al., Journal of Infection and Chemotherapy, doi:10.1016/j.jiac.2023.10.010, jRCT2041210004, Oct 2023
Early terminated RCT 84 patients in Japan, showing no significant difference in outcomes with favipiravir treatment. There was a trend for improved efficacy for patients enrolled within 48 hours of symptom onset.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
Standard of Care (SOC) for COVID-19 in the study country,
Japan, is very poor with very low average efficacy for approved treatments15.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
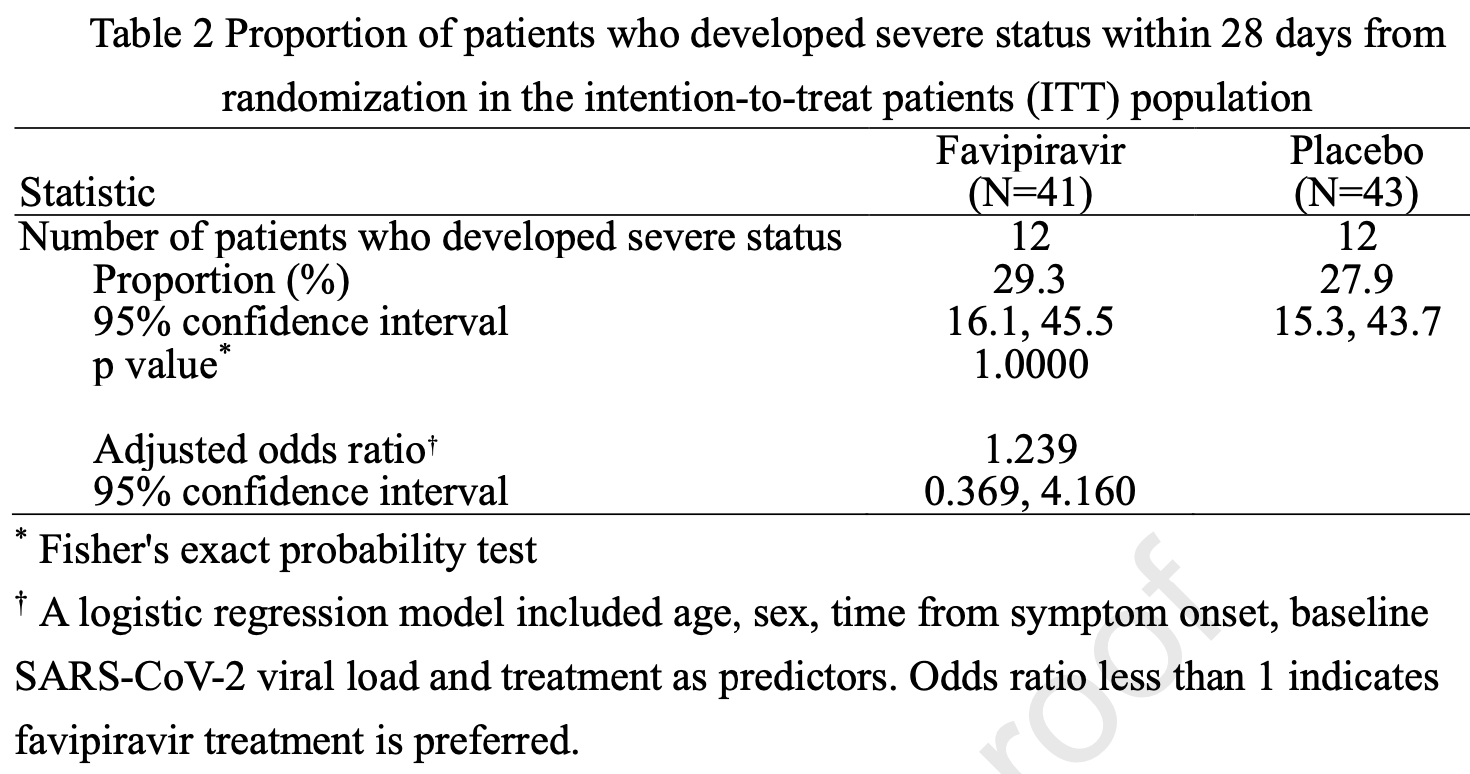
risk of oxygen therapy, 16.2% higher, RR 1.16, p = 0.73, treatment 12 of 43 (27.9%), control 12 of 43 (27.9%), adjusted per study, odds ratio converted to relative risk, multivariable, day 28.
|
|
risk of oxygen therapy, 18.5% lower, RR 0.81, p = 0.77, treatment 5 of 24 (20.8%), control 6 of 22 (27.3%), NNT 16, adjusted per study, odds ratio converted to relative risk, patients with onset ≤48 hours, multivariable, day 28.
|
|
risk of no viral clearance, 15.9% higher, RR 1.16, p = 0.66, treatment 21 of 41 (51.2%), control 19 of 43 (44.2%), day 15.
|
|
risk of no viral clearance, 6.0% lower, RR 0.94, p = 0.82, treatment 26 of 41 (63.4%), control 29 of 43 (67.4%), NNT 25, day 10.
|
|
risk of no viral clearance, 0.9% lower, RR 0.99, p = 1.00, treatment 34 of 41 (82.9%), control 36 of 43 (83.7%), NNT 126, day 7.
|
|
risk of no viral clearance, 5.6% lower, RR 0.94, p = 0.48, treatment 36 of 41 (87.8%), control 40 of 43 (93.0%), NNT 19, day 4.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
13.
Cenikli et al., Does Favipiravir interact with DNA? Design of electrochemical DNA nanobiosensor to investigate the interaction between DNA and Favipiravir used in the treatment of COVID-19, Talanta, doi:10.1016/j.talanta.2025.128084.
Iwata et al., 12 Oct 2023, Double Blind Randomized Controlled Trial, placebo-controlled, Japan, peer-reviewed, 13 authors, trial jRCT2041210004.
Contact: tsutomu.sakurai@fujifilm.com.
Findings from a discontinued clinical trial of favipiravir in high-risk patients with early-onset COVID-19
Journal of Infection and Chemotherapy, doi:10.1016/j.jiac.2023.10.010
Introduction: Favipiravir terminates severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) replication. Accordingly, early administration of favipiravir to SARS-CoV-2-infected coronavirus disease 2019 (COVID-19) patients may be expected to suppress disease progression. Methods: A randomized double-blind placebo-controlled trial was conducted to demonstrate efficacy of favipiravir in reducing disease progression in patients with mild COVID-19. The participants were unvaccinated patients with comorbidities and at risk of progression to severe disease. Patients were enrolled within 72 hours of disease onset and randomized to receive either favipiravir (1800 mg/dose on Day 1 followed by 800 mg/dose) or matching placebo twice daily for 10 days. The primary endpoint was the proportion of patients requiring oxygen therapy within 28 days of randomization.
Results: The trial was discontinued after enrolling 84 patients due to slower than anticipated enrollment caused by rapid uptake of SARS-CoV-2-vaccines and the emergence of the Omicron variant. Results from the 84 patients demonstrated no significant difference in all clinical outcomes. In post-hoc analyses, favipiravir treatment showed higher efficacy in patients within 48 hours of onset. No deaths or severe adverse events were documented in the favipiravir group. Plasma concentrations of favipiravir from Day 2 onward were maintained above 40 μg/mL.
Conclusions: Conducting clinical trials for pathogens like SARS-CoV-2 that rapidly accumulate mutations leading to altered disease characteristics carries significant risks unless it can be done in a short period. Therefore, it would be important to prepare the comprehensive clinical trial platform that can appropriately and promptly evaluate drugs even under a pandemic.
Declaration of competing interest TY and TS are employees of the sponsor. SI reports consulting fees from Fujifilm Toyama Chemical, and Meiji Seika Pharma; lecture fees from Pfizer, and Gilead Sciences. YD reports consulting fees from Fujifilm Toyama Chemical, Shionogi, GSK, Gilead Sciences, and Moderna. MS reports consulting fees from Genova Inc., Shionogi, Ono Pharmaceutical, AstraZeneca, Pfizer, Chugai, and Fujitsu; lecture fees from Fujifilm Toyama Chemical. OK, HK, and KT report consulting fee from Fujifilm Toyama Chemical. KK, MY, AK, and TO report lecture fee from Fujifilm Toyama Chemical. J o u r n a l P r e -p r o o f
References
Blair, Nirmatrelvir plus ritonavir in COVID-19: a profile of its use, Drugs Ther Perspect, doi:10.1007/s40267-022-00971-1
Chiba, Kiso, Nakajima, Iida, Maemura et al., Coadministration of favipiravir and the remdesivir metabolite GS-441524 effectively reduces SARS-CoV-2 replication in the lungs of the syrian hamster model, mBio, doi:10.1128/mbio.03044-21
Doi, Ishihara, Banno, Ando, Kondo et al., Favipiravir for symptomatic COVID-19: A nationwide observational cohort study, J Infect Chemother, doi:10.1016/j.jiac.2022.10.008
Driouich, Cochin, Lingas, Moureau, Touret et al., Favipiravir antiviral efficacy against SARS-CoV-2 in a hamster model, Nat Commun, doi:10.1038/s41467-021-21992-w
Gowen, Wong, Jung, Smee, Morrey et al., Efficacy of favipiravir (T-705) and T-1106 pyrazine derivatives in phlebovirus disease models, Antiviral Res, doi:10.1016/j.antiviral.2009.10.015
He, Lau, Wu, Deng, Wang et al., Temporal dynamics in viral shedding and transmissibility of COVID-19, Nat Med, doi:10.1038/s41591-020-0869-5
Kaptein, Jacobs, Langendries, Seldeslachts, Horst et al., -p r o o f al. Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity, Proc Natl Acad Sci U S A, doi:10.1073/pnas.2014441117
Killingley, Mann, Kalinova, Boyers, Goonawardane et al., Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults, Nat Med, doi:10.1038/s41591-022-01780-9
Madhi, Kwatra, Myers, Jassat, Dhar et al., Population immunity and Covid-19 severity with Omicron variant in South Africa, N Engl J Med. J o u r n a l P r e -p r o o f, doi:10.1056/NEJMoa2119658
Noor, Islam, Prevalence and associated risk factors of mortality among COVID-19 patients: a meta-analysis, J Community Health, doi:10.1007/s10900-020-00920-x
Oestereich, Lüdtke, Wurr, Rieger, Muñoz-Fontela et al., Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model, Antiviral Res, doi:10.1016/j.antiviral.2014.02.014
Oestereich, Rieger, Lüdtke, Ruibal, Wurr et al., Efficacy of favipiravir alone and in combination with ribavirin in a lethal, immunocompetent mouse model of Lassa fever, J Infect Dis, doi:10.1093/infdis/jiv522
Ogata, Tanaka, Irie, Hirayama, Takahashi, Shorter incubation period among unvaccinated Delta variant Coronavirus Disease 2019 Patients in Japan, Int J Environ Res Public Health, doi:10.3390/ijerph19031127
Ohashi, Watashi, Saso, Shionoya, Iwanami et al., Potential anti-COVID-19 agents, cepharanthine and nelfinavir, and their usage for combination treatment, iScience, doi:10.1016/j.isci.2021.102367
Shinkai, Tsushima, Tanaka, Hagiwara, Tarumoto et al., Efficacy and safety of favipiravir in moderate COVID-19 pneumonia patients without oxygen therapy: A randomized, phase III clinical trial, Infect Dis Ther, doi:10.1007/s40121-021-00517-4
Singh, Khera, Chugh, Khera, Chugh, Efficacy and safety of remdesivir in COVID-19 caused by SARS-CoV-2: a systematic review and meta-analysis, BMJ Open, doi:10.1136/bmjopen-2020-048416
Singh, Singh, Singh, Misra, An updated practical guideline on use of molnupiravir and comparison with agents having emergency use authorization for treatment of COVID-19, Diabetes Metab Syndr, doi:10.1016/j.dsx.2022.102396
Sirijatuphat, Manosuthi, Niyomnaitham, Owen, Copeland et al., Early treatment of favipiravir in COVID-19 patients without pneumonia: a multicentre, open-labelled, randomized control study, Emerg Microbes Infect, doi:10.1080/22221751.2022.2117092
Sissoko, Laouenan, Folkesson, Lebing, Beavogui et al., Experimental treatment with favipiravir for Ebola virus disease (the JIKI trial): A historically controlled, single-arm proof-of-concept trial in Guinea, PLoS Med, doi:10.1371/journal.pmed.1001967
Tanaka, Ogata, Shibata, Nagai, Takahashi et al., Shorter incubation period among COVID-19 cases with the BA.1 Omicron variant, Int J Environ Res Public Health, doi:10.3390/ijerph19106330
Udwadia, Singh, Barkate, Patil, Rangwala et al., Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-tomoderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial, Int J Infect Dis, doi:10.1016/j.ijid.2020.11.142
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res, doi:10.1038/s41422-020-0282-0
Yotsuyanagi, Ohmagari, Doi, Imamura, Sonoyama et al., A phase 2/3 study of S-217622 in participants with SARS-CoV-2 infection (Phase 3 part), Medicine, doi:10.1097/MD.0000000000033024
DOI record:
{
"DOI": "10.1016/j.jiac.2023.10.010",
"ISSN": [
"1341-321X"
],
"URL": "http://dx.doi.org/10.1016/j.jiac.2023.10.010",
"alternative-id": [
"S1341321X23002556"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Findings from a discontinued clinical trial of favipiravir in high-risk patients with early-onset COVID-19"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Infection and Chemotherapy"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jiac.2023.10.010"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 Japanese Society of Chemotherapy, Japanese Association for Infectious Diseases, and Japanese Society for Infection Prevention and Control. Published by Elsevier Ltd."
}
],
"author": [
{
"affiliation": [],
"family": "Iwata",
"given": "Satoshi",
"sequence": "first"
},
{
"affiliation": [],
"family": "Kobayashi",
"given": "Osamu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kurashima",
"given": "Kazuyoshi",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9620-2525",
"affiliation": [],
"authenticated-orcid": false,
"family": "Doi",
"given": "Yohei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kunishima",
"given": "Hiroyuki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shinkai",
"given": "Masaharu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tsushima",
"given": "Kenji",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yamato",
"given": "Masaya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kano",
"given": "Akira",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1638-5027",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hibino",
"given": "Makoto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yamatake",
"given": "Takahiro",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6496-4840",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sakurai",
"given": "Tsutomu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ogura",
"given": "Takashi",
"sequence": "additional"
}
],
"container-title": "Journal of Infection and Chemotherapy",
"container-title-short": "Journal of Infection and Chemotherapy",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"jiac-j.com",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
10,
12
]
],
"date-time": "2023-10-12T02:03:35Z",
"timestamp": 1697076215000
},
"deposited": {
"date-parts": [
[
2023,
10,
12
]
],
"date-time": "2023-10-12T06:18:13Z",
"timestamp": 1697091493000
},
"indexed": {
"date-parts": [
[
2023,
10,
13
]
],
"date-time": "2023-10-13T05:33:28Z",
"timestamp": 1697175208455
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
10
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
10,
1
]
],
"date-time": "2023-10-01T00:00:00Z",
"timestamp": 1696118400000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 11,
"start": {
"date-parts": [
[
2023,
10,
12
]
],
"date-time": "2023-10-12T00:00:00Z",
"timestamp": 1697068800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1341321X23002556?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1341321X23002556?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
10
]
]
},
"published-print": {
"date-parts": [
[
2023,
10
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1136/bmjopen-2020-048416",
"article-title": "Efficacy and safety of remdesivir in COVID-19 caused by SARS-CoV-2: a systematic review and meta-analysis",
"author": "Singh",
"doi-asserted-by": "crossref",
"journal-title": "BMJ Open",
"key": "10.1016/j.jiac.2023.10.010_bib1",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1016/j.dsx.2022.102396",
"article-title": "An updated practical guideline on use of molnupiravir and comparison with agents having emergency use authorization for treatment of COVID-19",
"author": "Singh",
"doi-asserted-by": "crossref",
"journal-title": "Diabetes Metabol Syndr",
"key": "10.1016/j.jiac.2023.10.010_bib2",
"volume": "16",
"year": "2022"
},
{
"article-title": "Nirmatrelvir plus ritonavir in COVID-19: a profile of its use",
"author": "Blair",
"first-page": "1",
"journal-title": "Drugs Ther Perspect",
"key": "10.1016/j.jiac.2023.10.010_bib3",
"year": "2022"
},
{
"DOI": "10.1097/MD.0000000000033024",
"article-title": "A phase 2/3 study of S-217622 in participants with SARS-CoV-2 infection (Phase 3 part)",
"author": "Yotsuyanagi",
"doi-asserted-by": "crossref",
"journal-title": "Medicine (Baltim)",
"key": "10.1016/j.jiac.2023.10.010_bib4",
"volume": "102",
"year": "2023"
},
{
"DOI": "10.1093/infdis/jiv522",
"article-title": "Efficacy of favipiravir alone and in combination with ribavirin in a lethal, immunocompetent mouse model of Lassa fever",
"author": "Oestereich",
"doi-asserted-by": "crossref",
"first-page": "934",
"journal-title": "J Infect Dis",
"key": "10.1016/j.jiac.2023.10.010_bib6",
"volume": "213",
"year": "2016"
},
{
"DOI": "10.1016/j.antiviral.2009.10.015",
"article-title": "Efficacy of favipiravir (T-705) and T-1106 pyrazine derivatives in phlebovirus disease models",
"author": "Gowen",
"doi-asserted-by": "crossref",
"first-page": "121",
"journal-title": "Antivir Res",
"key": "10.1016/j.jiac.2023.10.010_bib7",
"volume": "86",
"year": "2010"
},
{
"DOI": "10.1016/j.antiviral.2014.02.014",
"article-title": "Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model",
"author": "Oestereich",
"doi-asserted-by": "crossref",
"first-page": "17",
"journal-title": "Antivir Res",
"key": "10.1016/j.jiac.2023.10.010_bib8",
"volume": "105",
"year": "2014"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "269",
"journal-title": "Cell Res",
"key": "10.1016/j.jiac.2023.10.010_bib9",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1016/j.isci.2021.102367",
"article-title": "Potential anti-COVID-19 agents, cepharanthine and nelfinavir, and their usage for combination treatment",
"author": "Ohashi",
"doi-asserted-by": "crossref",
"issue": "4",
"journal-title": "iScience",
"key": "10.1016/j.jiac.2023.10.010_bib10",
"volume": "24",
"year": "2021"
},
{
"DOI": "10.1038/s41467-021-21992-w",
"article-title": "Favipiravir antiviral efficacy against SARS-CoV-2 in a hamster model",
"author": "Driouich",
"doi-asserted-by": "crossref",
"first-page": "1735",
"journal-title": "Nat Commun",
"key": "10.1016/j.jiac.2023.10.010_bib11",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1073/pnas.2014441117",
"article-title": "Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity",
"author": "Kaptein",
"doi-asserted-by": "crossref",
"first-page": "26955",
"issue": "43",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "10.1016/j.jiac.2023.10.010_bib12",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1128/mbio.03044-21",
"article-title": "Co-administration of favipiravir and the remdesivir metabolite GS-441524 effectively reduces SARS-CoV-2 replication in the lungs of the syrian hamster model",
"author": "Chiba",
"doi-asserted-by": "crossref",
"journal-title": "mBio",
"key": "10.1016/j.jiac.2023.10.010_bib13",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1007/s40121-021-00517-4",
"article-title": "Efficacy and safety of favipiravir in moderate COVID-19 pneumonia patients without oxygen therapy: a randomized, phase III clinical trial",
"author": "Shinkai",
"doi-asserted-by": "crossref",
"first-page": "2489",
"journal-title": "Infect Dis Ther",
"key": "10.1016/j.jiac.2023.10.010_bib14",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1038/s41591-020-0869-5",
"article-title": "Temporal dynamics in viral shedding and transmissibility of COVID-19",
"author": "He",
"doi-asserted-by": "crossref",
"first-page": "672",
"journal-title": "Nat Med",
"key": "10.1016/j.jiac.2023.10.010_bib15",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1007/s10900-020-00920-x",
"article-title": "Prevalence and associated risk factors of mortality among COVID-19 patients: a meta-analysis",
"author": "Noor",
"doi-asserted-by": "crossref",
"first-page": "1270",
"journal-title": "J Community Health",
"key": "10.1016/j.jiac.2023.10.010_bib16",
"volume": "45",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.11.142",
"article-title": "Efficacy and safety of favipiravir, an oral RNA-dependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: a randomized, comparative, open-label, multicenter, phase 3 clinical trial",
"author": "Udwadia",
"doi-asserted-by": "crossref",
"first-page": "62",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.jiac.2023.10.010_bib17",
"volume": "103",
"year": "2021"
},
{
"DOI": "10.1080/22221751.2022.2117092",
"article-title": "Early treatment of favipiravir in COVID-19 patients without pneumonia: a multicentre, open-labelled, randomized control study",
"author": "Sirijatuphat",
"doi-asserted-by": "crossref",
"first-page": "2197",
"journal-title": "Emerg Microb Infect",
"key": "10.1016/j.jiac.2023.10.010_bib18",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1371/journal.pmed.1001967",
"article-title": "Experimental treatment with favipiravir for Ebola virus disease (the JIKI trial): a historically controlled, single-arm proof-of-concept trial in Guinea",
"author": "Sissoko",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "PLoS Med",
"key": "10.1016/j.jiac.2023.10.010_bib19",
"volume": "13",
"year": "2016"
},
{
"DOI": "10.1016/j.jiac.2022.10.008",
"article-title": "Favipiravir for symptomatic COVID-19: a nationwide observational cohort study",
"author": "Doi",
"doi-asserted-by": "crossref",
"first-page": "150",
"issue": "2",
"journal-title": "J Infect Chemother",
"key": "10.1016/j.jiac.2023.10.010_bib20",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2119658",
"article-title": "Population immunity and Covid-19 severity with Omicron variant in South Africa",
"author": "Madhi",
"doi-asserted-by": "crossref",
"first-page": "1314",
"issue": "14",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiac.2023.10.010_bib22",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1038/s41591-022-01780-9",
"article-title": "Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults",
"author": "Killingley",
"doi-asserted-by": "crossref",
"first-page": "1031",
"journal-title": "Nat Med",
"key": "10.1016/j.jiac.2023.10.010_bib23",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.3390/ijerph19031127",
"article-title": "Shorter incubation period among unvaccinated Delta variant Coronavirus Disease 2019 Patients in Japan",
"author": "Ogata",
"doi-asserted-by": "crossref",
"first-page": "1127",
"issue": "3",
"journal-title": "Int J Environ Res Publ Health",
"key": "10.1016/j.jiac.2023.10.010_bib24",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.3390/ijerph19106330",
"article-title": "Shorter incubation period among COVID-19 cases with the BA.1 Omicron variant",
"author": "Tanaka",
"doi-asserted-by": "crossref",
"first-page": "6330",
"issue": "10",
"journal-title": "Int J Environ Res Publ Health",
"key": "10.1016/j.jiac.2023.10.010_bib25",
"volume": "19",
"year": "2022"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1341321X23002556"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Pharmacology (medical)",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Findings from a discontinued clinical trial of favipiravir in high-risk patients with early-onset COVID-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}