Real-world experience with molnupiravir during the period of SARS-CoV-2 Omicron variant dominance
et al., Pharmacological Reports, doi:10.1007/s43440-022-00408-6, Jul 2022 (preprint)
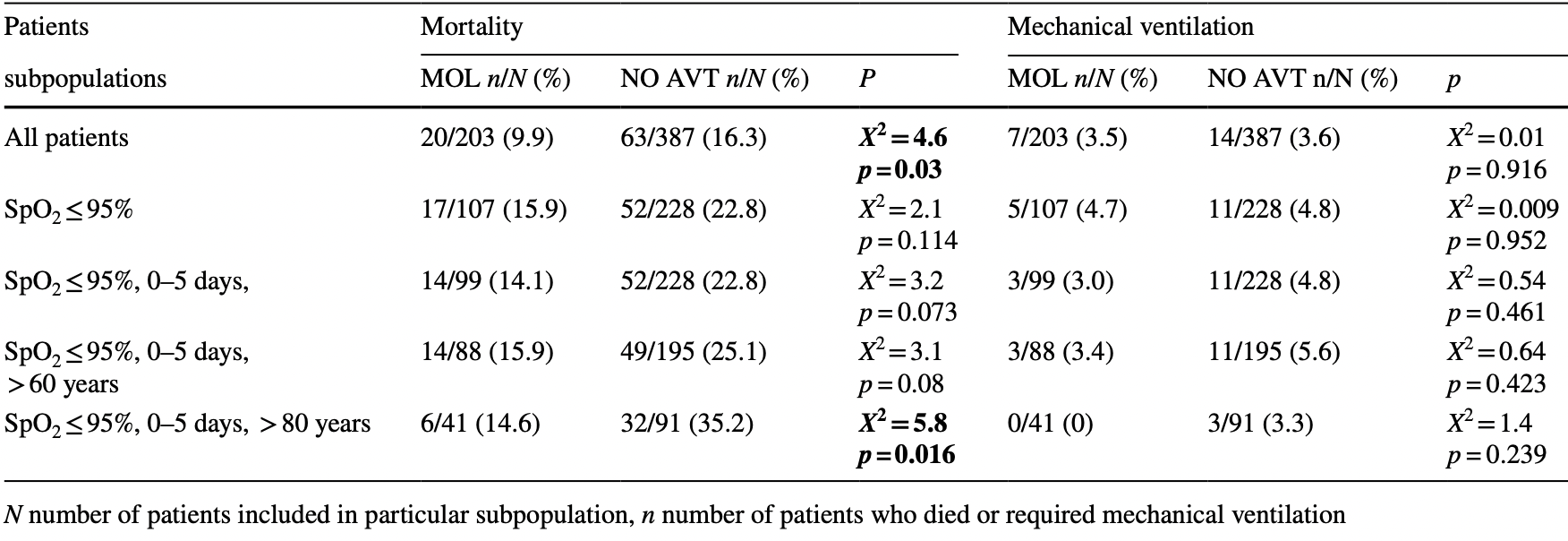
Retrospective 590 patients in Poland, 203 treated with mulnupiravir, showing lower mortality with treatment.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
|
risk of death, 39.5% lower, RR 0.61, p = 0.03, treatment 20 of 203 (9.9%), control 63 of 387 (16.3%), NNT 16.
|
|
risk of mechanical ventilation, 4.7% lower, RR 0.95, p = 1.00, treatment 7 of 203 (3.4%), control 14 of 387 (3.6%), NNT 591.
|
|
hospitalization time, 0.9% higher, relative time 1.01, p = 0.96, treatment mean 11.6 (±7.9) n=203, control mean 11.5 (±9.3) n=387.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
Flisiak et al., 6 Jul 2022, retrospective, Poland, peer-reviewed, 13 authors, study period 1 January, 2022 - 30 April, 2022.
Contact: robert.flisiak1@gmail.com.
Real-world experience with molnupiravir during the period of SARS-CoV-2 Omicron variant dominance
Pharmacological Reports, doi:10.1007/s43440-022-00408-6
Background The real-world effectiveness of molnupiravir (MOL) during the dominance of Omicron SARS-CoV-2 lineage is urgently needed since the available data relate to the period of circulation of other viral variants. Therefore, this study assessed the efficacy of MOL in patients hospitalized for COVID-19 in a real-world clinical practice during the wave of Omicron infections. Methods Among 11,822 patients hospitalized after 1 March 2020 and included in the SARSTer national database, 590 were treated between 1 January and 31 April 2022, a period of dominance of the Omicron SARS-CoV-2 variant. MOL was administered to 203 patients, whereas 387 did not receive any antiviral regimen. Both groups were similar in terms of sex, BMI and age allowing for direct comparisons. Results Patients who did not receive antiviral therapy significantly more often required the use of Dexamethasone and Baricitinib. Treatment with MOL resulted in a statistically significant reduction in mortality during the 28-day follow-up (9.9 vs. 16.3%), which was particularly evident in the population of patients over 80 years of age treated in the first 5 days of the disease (14.6 vs. 35.2%). MOL therapy did not affect the frequency of the need for mechanical ventilation, but patients treated with MOL required oxygen supplementation less frequently than those without antivirals (31.7 vs. 49.2%). The time of hospitalization did not differ between groups. Conclusions The use of molnupiravir in patients hospitalized for COVID-19 during the dominance of Omicron variant reduced mortality. This effect is particularly evident in patients over 80 years of age.
References
Arribas, Bhagani, Lobo, Khaertynova, Mateu et al., Randomized trial of molnupiravir or placebo in patients hospitalized with covid-19, NEJM Evid, doi:10.1056/evidoa2100044
Flisiak, Horban, Jaroszewicz, Kozielewicz, Mastalerz-Migas et al., Diagnosis and therapy of SARS-CoV-2 infection: recommendations of the Polish Association of Epidemiologists and Infectiologists as of November 12, 2021. Annex no. 1 to the Recommendations of April 26, 2021, Pol Arch Intern Med, doi:10.20452/pamw.16140
Flisiak, Horban, Jaroszewicz, Kozielewicz, Mastalerz-Migas et al., Management of SARS-CoV-2 infection: recommendations of the Polish Association of Epidemiologists and Infectiologists as of February 23, Pol Arch Intern Med, doi:10.20452/pamw.16230
Flisiak, Zarębska-Michaluk, Berkan-Kawińska, Tudrujek-Zdunek, Rogalska et al., Remdesivir-based therapy improved the recovery of patients with COVID-19 in the multicenter, real-world SARSTer study, Pol Arch Intern Med
Gordon, Tchesnokov, Schinazi, Götte, Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template, J Biol Chem
Jayk, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients, N Engl J Med
Kumarasamy, Jindal, Saha, Singh, Rodduturi et al., Phase III trial of molnupiravir in adults with mild SARS-cov2 infection in India
Mader, Tydykov, Glück, Bertok, Weidlich et al., Omicron's binding to sotrovimab, casirivimab, imdevimab, CR3022, and sera from previously infected or vaccinated individuals, IScience
Painter, Natchus, Cohen, Holman, Painter, Developing a direct acting, orally available antiviral agent in a pandemic: the evolution of molnupiravir as a potential treatment for COVID-19, Curr Opin Virol
Rahmah, Abarikwu, Arero, Jibril, Fal et al., Oral antiviral treatments for COVID-19: opportunities and challenges, Rep: Pharmacol, doi:10.1007/s43440-022-00388-7
Vangeel, Chiu, Jonghe, Maes, Slechten et al., Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern, Antiviral Res
Wahl, Gralinski, Johnson, Yao, Kovarova et al., SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801, Nature
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of molnupiravir and nirmatrelvir/ ritonavir among COVID-19 inpatients during Hong Kong's Omicron BA.2 wave: an observational study, doi:10.1101/2022.05.19.22275291
Zarębska-Michaluk, Jaroszewicz, Rogalska, Martonik, Pabjan et al., Effectiveness of tocilizumab with and without dexamethasone in patients with severe COVID-19: a retrospective study, J Inflamm Res
DOI record:
{
"DOI": "10.1007/s43440-022-00408-6",
"ISSN": [
"1734-1140",
"2299-5684"
],
"URL": "http://dx.doi.org/10.1007/s43440-022-00408-6",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>The real-world effectiveness of molnupiravir (MOL) during the dominance of Omicron SARS-CoV-2 lineage is urgently needed since the available data relate to the period of circulation of other viral variants. Therefore, this study assessed the efficacy of MOL in patients hospitalized for COVID-19 in a real-world clinical practice during the wave of Omicron infections.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>Among 11,822 patients hospitalized after 1 March 2020 and included in the SARSTer national database, 590 were treated between 1 January and 31 April 2022, a period of dominance of the Omicron SARS-CoV-2 variant. MOL was administered to 203 patients, whereas 387 did not receive any antiviral regimen. Both groups were similar in terms of sex, BMI and age allowing for direct comparisons.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Patients who did not receive antiviral therapy significantly more often required the use of Dexamethasone and Baricitinib. Treatment with MOL resulted in a statistically significant reduction in mortality during the 28-day follow-up (9.9 vs. 16.3%), which was particularly evident in the population of patients over 80 years of age treated in the first 5 days of the disease (14.6 vs. 35.2%). MOL therapy did not affect the frequency of the need for mechanical ventilation, but patients treated with MOL required oxygen supplementation less frequently than those without antivirals (31.7 vs. 49.2%). The time of hospitalization did not differ between groups.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>The use of molnupiravir in patients hospitalized for COVID-19 during the dominance of Omicron variant reduced mortality. This effect is particularly evident in patients over 80 years of age.</jats:p>\n </jats:sec>",
"alternative-id": [
"408"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "28 June 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Revised",
"name": "revised",
"order": 2,
"value": "12 August 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 3,
"value": "17 August 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 4,
"value": "24 August 2022"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Robert Flisiak and Jerzy Jaroszewicz reports grants and personal fees from MSD, Gilead, and Roche; Dorota Zarębska-Michaluk reports personal fees from MSD and Gilead; Justyna Kowalska reports grants and personal fees from MSD, Gilead, Janssen Cilag, GSK/ViiV and Roche; Anna Moniuszko-Malinowska reports personal fee from DiaSorin; Regina Podlasin reports personal fees from Gilead; Magdalena Rogalska, Justyna Anna Kryńska, Ewa Dutkiewicz, Krystyna Dobrowolska, Marta Rorat, Olga Tronina, Piotr Rzymski report no financial interests in relation to the work described."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-3394-1635",
"affiliation": [],
"authenticated-orcid": false,
"family": "Flisiak",
"given": "Robert",
"sequence": "first"
},
{
"affiliation": [],
"family": "Zarębska-Michaluk",
"given": "Dorota",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rogalska",
"given": "Magdalena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kryńska",
"given": "Justyna Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kowalska",
"given": "Justyna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dutkiewicz",
"given": "Ewa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dobrowolska",
"given": "Krystyna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jaroszewicz",
"given": "Jerzy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moniuszko-Malinowska",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rorat",
"given": "Marta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Podlasin",
"given": "Regina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tronina",
"given": "Olga",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rzymski",
"given": "Piotr",
"sequence": "additional"
}
],
"container-title": "Pharmacological Reports",
"container-title-short": "Pharmacol. Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
8,
24
]
],
"date-time": "2022-08-24T13:02:15Z",
"timestamp": 1661346135000
},
"deposited": {
"date-parts": [
[
2022,
8,
24
]
],
"date-time": "2022-08-24T13:03:53Z",
"timestamp": 1661346233000
},
"funder": [
{
"name": "Polskie Towarzystwo Epidemiologów i Lekarzy Chorób Zakaźnych"
}
],
"indexed": {
"date-parts": [
[
2022,
8,
25
]
],
"date-time": "2022-08-25T04:09:56Z",
"timestamp": 1661400596990
},
"is-referenced-by-count": 1,
"issued": {
"date-parts": [
[
2022,
8,
24
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
8,
24
]
],
"date-time": "2022-08-24T00:00:00Z",
"timestamp": 1661299200000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
8,
24
]
],
"date-time": "2022-08-24T00:00:00Z",
"timestamp": 1661299200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s43440-022-00408-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s43440-022-00408-6/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s43440-022-00408-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1007",
"published": {
"date-parts": [
[
2022,
8,
24
]
]
},
"published-online": {
"date-parts": [
[
2022,
8,
24
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/j.isci.2022.104076",
"author": "A-L Mader",
"doi-asserted-by": "publisher",
"journal-title": "IScience",
"key": "408_CR1",
"unstructured": "Mader A-L, Tydykov L, Glück V, Bertok M, Weidlich T, Gottwald C, et al. Omicron’s binding to sotrovimab, casirivimab, imdevimab, CR3022, and sera from previously infected or vaccinated individuals. IScience. 2022;25: 104076.",
"volume": "25",
"year": "2022"
},
{
"DOI": "10.1016/j.antiviral.2022.105252",
"author": "L Vangeel",
"doi-asserted-by": "publisher",
"journal-title": "Antiviral Res",
"key": "408_CR2",
"unstructured": "Vangeel L, Chiu W, De Jonghe S, Maes P, Slechten B, Raymenants J, et al. Remdesivir, molnupiravir and nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern. Antiviral Res. 2022;198: 105252.",
"volume": "198",
"year": "2022"
},
{
"DOI": "10.1007/s43440-022-00388-7",
"doi-asserted-by": "publisher",
"key": "408_CR3",
"unstructured": "Rahmah L, Abarikwu SO, Arero AG, Jibril AT, Fal A, Flisiak R, et al. Oral antiviral treatments for COVID-19: opportunities and challenges. Rep: Pharmacol; 2022. https://doi.org/10.1007/s43440-022-00388-7."
},
{
"DOI": "10.1016/j.coviro.2021.06.003",
"author": "GR Painter",
"doi-asserted-by": "publisher",
"first-page": "17",
"journal-title": "Curr Opin Virol",
"key": "408_CR4",
"unstructured": "Painter GR, Natchus MG, Cohen O, Holman W, Painter WP. Developing a direct acting, orally available antiviral agent in a pandemic: the evolution of molnupiravir as a potential treatment for COVID-19. Curr Opin Virol. 2021;50:17–22.",
"volume": "50",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03312-w",
"author": "A Wahl",
"doi-asserted-by": "publisher",
"first-page": "451",
"journal-title": "Nature",
"key": "408_CR5",
"unstructured": "Wahl A, Gralinski LE, Johnson CE, Yao W, Kovarova M, Dinnon KH 3rd, et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature. 2021;591:451–7.",
"volume": "591",
"year": "2021"
},
{
"key": "408_CR6",
"unstructured": "Kumarasamy N, Jindal, A, Saha, B, Singh VB, Rodduturi NCR, Sinha S, Sriramadasu SC. Phase III trial of molnupiravir in adults with mild SARS-cov2 infection in India. In: CROI Virtual Conference, Denver 2022, abstract #101"
},
{
"DOI": "10.1016/j.jbc.2021.100770",
"author": "CJ Gordon",
"doi-asserted-by": "publisher",
"journal-title": "J Biol Chem",
"key": "408_CR7",
"unstructured": "Gordon CJ, Tchesnokov EP, Schinazi RF, Götte M. Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template. J Biol Chem. 2021;297: 100770.",
"volume": "297",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116044",
"author": "BA Jayk",
"doi-asserted-by": "publisher",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "408_CR8",
"unstructured": "Jayk BA, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med. 2022;386:509–20.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/evidoa2100044",
"author": "JR Arribas",
"doi-asserted-by": "publisher",
"journal-title": "NEJM Evid",
"key": "408_CR9",
"unstructured": "Arribas JR, Bhagani S, Lobo SM, Khaertynova I, Mateu L, Fishchuk R, et al. Randomized trial of molnupiravir or placebo in patients hospitalized with covid-19. NEJM Evid. 2022. https://doi.org/10.1056/evidoa2100044.",
"year": "2022"
},
{
"DOI": "10.20452/pamw.16140",
"author": "R Flisiak",
"doi-asserted-by": "publisher",
"first-page": "487",
"journal-title": "Pol Arch Intern Med",
"key": "408_CR10",
"unstructured": "Flisiak R, Horban A, Jaroszewicz J, Kozielewicz D, Mastalerz-Migas A, Owczuk R, et al. Diagnosis and therapy of SARS-CoV-2 infection: recommendations of the Polish Association of Epidemiologists and Infectiologists as of November 12, 2021. Annex no. 1 to the Recommendations of April 26, 2021. Pol Arch Intern Med. 2021;131:487–96. https://doi.org/10.20452/pamw.16140.",
"volume": "131",
"year": "2021"
},
{
"DOI": "10.20452/pamw.16230",
"author": "R Flisiak",
"doi-asserted-by": "publisher",
"journal-title": "Pol Arch Intern Med",
"key": "408_CR11",
"unstructured": "Flisiak R, Horban A, Jaroszewicz J, Kozielewicz D, Mastalerz-Migas A, Owczuk R, et al. Management of SARS-CoV-2 infection: recommendations of the Polish Association of Epidemiologists and Infectiologists as of February 23, 2022. Pol Arch Intern Med. 2022. https://doi.org/10.20452/pamw.16230.",
"year": "2022"
},
{
"key": "408_CR12",
"unstructured": "MI2DataLab. Monitor of SARS-CoV-2. 2022. https://monitor.crs19.pl/2022-03-31/poland/?lang=en. Accessed June 23 2022."
},
{
"author": "R Flisiak",
"first-page": "103",
"journal-title": "Pol Arch Intern Med",
"key": "408_CR13",
"unstructured": "Flisiak R, Zarębska-Michaluk D, Berkan-Kawińska A, Tudrujek-Zdunek M, Rogalska M, Piekarska A, et al. Remdesivir-based therapy improved the recovery of patients with COVID-19 in the multicenter, real-world SARSTer study. Pol Arch Intern Med. 2021;131:103–10.",
"volume": "131",
"year": "2021"
},
{
"DOI": "10.2147/JIR.S322645",
"author": "D Zarębska-Michaluk",
"doi-asserted-by": "publisher",
"first-page": "3359",
"journal-title": "J Inflamm Res",
"key": "408_CR14",
"unstructured": "Zarębska-Michaluk D, Jaroszewicz J, Rogalska M, Martonik D, Pabjan P, Berkan-Kawińska A, et al. Effectiveness of tocilizumab with and without dexamethasone in patients with severe COVID-19: a retrospective study. J Inflamm Res. 2021;14:3359–66.",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1101/2022.05.19.22275291",
"author": "CKH Wong",
"doi-asserted-by": "publisher",
"journal-title": "BioRxiv",
"key": "408_CR15",
"unstructured": "Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of molnupiravir and nirmatrelvir/ritonavir among COVID-19 inpatients during Hong Kong’s Omicron BA.2 wave: an observational study. BioRxiv. 2022. https://doi.org/10.1101/2022.05.19.22275291.",
"year": "2022"
}
],
"reference-count": 15,
"references-count": 15,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s43440-022-00408-6"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology",
"General Medicine"
],
"subtitle": [],
"title": "Real-world experience with molnupiravir during the period of SARS-CoV-2 Omicron variant dominance",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy"
}