US201 Study: A Phase 2, Randomized Proof-of-Concept Trial of Favipiravir for the Treatment of COVID-19
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofab56310, NCT04358549, Dec 2021
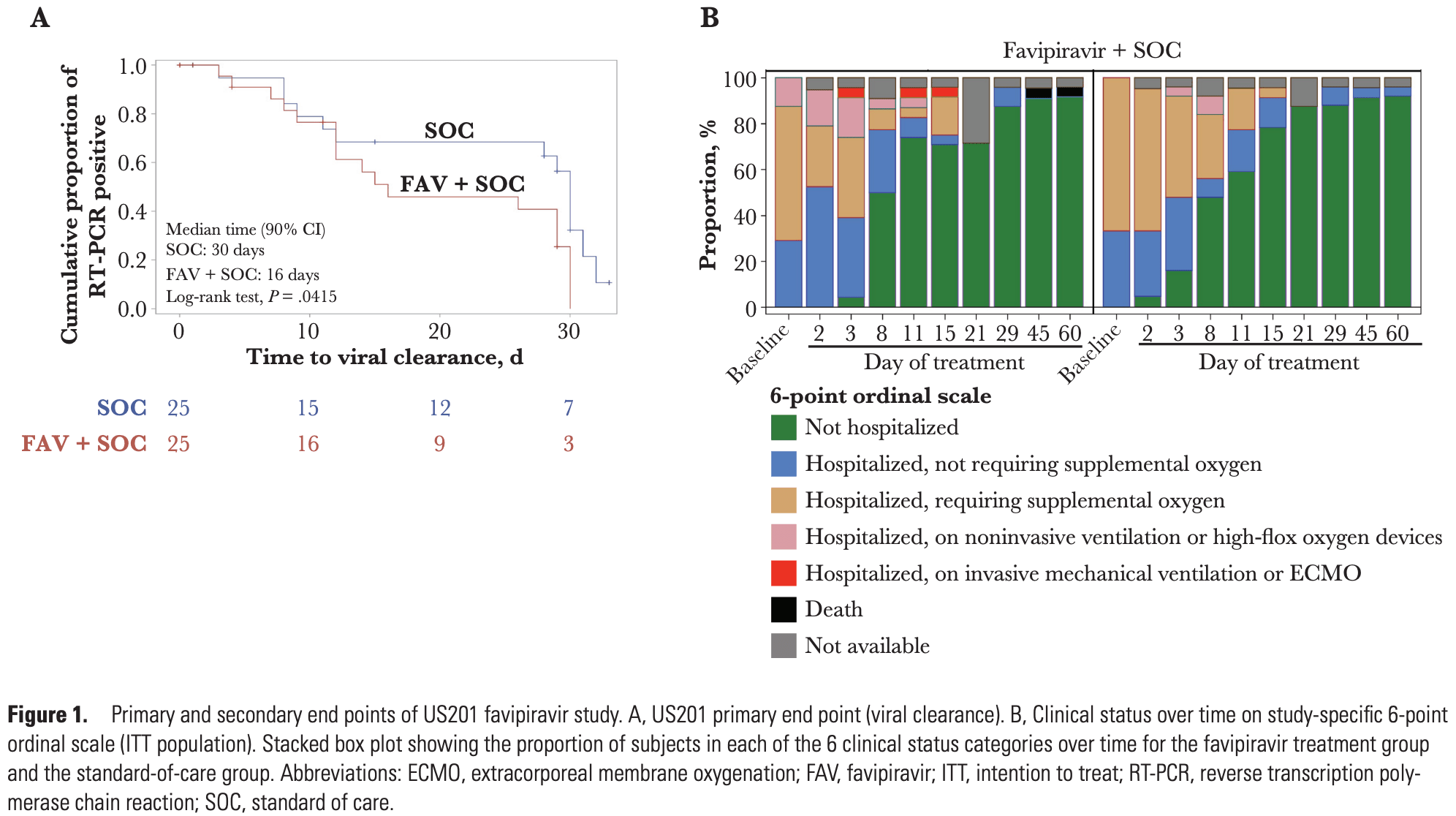
Small very late treatment RCT in the USA, with 25 favipiravir and 25 control patients, showing faster viral clearance with treatment. The benefit was only seen in patients <8 days from symptom onset. There were no significant differences in clinical outcomes. The death in the favipiravir group occurred after discharge and was believed to be unrelated to COVID-19 or favipiravir.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments15.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 200.0% higher, RR 3.00, p = 1.00, treatment 1 of 25 (4.0%), control 0 of 25 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), day 60.
|
|
risk of mechanical ventilation, 200.0% higher, RR 3.00, p = 1.00, treatment 1 of 25 (4.0%), control 0 of 25 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
hospitalization time, 19.8% higher, relative time 1.20, treatment 25, control 25.
|
|
risk of no recovery, 58.1% lower, OR 0.42, p = 0.08, treatment 25, control 25, inverted to make OR<1 favor treatment, day 8 mid-recovery, 6-point ordinal scale, RR approximated with OR.
|
|
risk of no recovery, 46.2% higher, OR 1.46, p = 0.54, treatment 25, control 25, inverted to make OR<1 favor treatment, day 15, 6-point ordinal scale, RR approximated with OR.
|
|
recovery time, 42.9% lower, relative time 0.57, treatment 25, control 25, median time to aggregate NEWS2 score ≤2 or discharge.
|
|
recovery time, 15.4% higher, relative time 1.15, treatment 25, control 25.
|
|
time to viral-, 46.7% lower, relative time 0.53, p = 0.04, treatment 25, control 25, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
13.
Cenikli et al., Does Favipiravir interact with DNA? Design of electrochemical DNA nanobiosensor to investigate the interaction between DNA and Favipiravir used in the treatment of COVID-19, Talanta, doi:10.1016/j.talanta.2025.128084.
Finberg et al., 7 Dec 2021, Randomized Controlled Trial, USA, peer-reviewed, 10 authors, study period 17 April, 2020 - 30 October, 2020, average treatment delay 8.4 days, trial NCT04358549 (history).
US201 Study: A Phase 2, Randomized Proof-of-Concept Trial of Favipiravir for the Treatment of COVID-19
Open Forum Infectious Diseases, doi:10.1093/ofid/ofab563
Background. Favipiravir is used to treat influenza, and studies demonstrate that it has antiviral activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Methods. We performed a randomized, open-label, multicenter, phase 2 proof-of-concept trial of favipiravir in hospitalized adult patients with polymerase chain reaction (PCR)-positive coronavirus disease 2019 . Patients were randomized to standard of care (SOC) or favipiravir treatment (1800 mg per os twice a day [b.i.d.] on day 1, followed by 1000 mg b.i.d. for 13 days). The primary end point was time to viral clearance on day 29. Results. Fifty patients were enrolled and stratified by disease severity (critical disease, severe disease, or mild to moderate disease). Nineteen patients were censored from the event of viral clearance based on being SARS-CoV-2 PCR-negative at the study outset, being PCR-positive at day 29, or because of loss to follow-up. Data from the 31 remaining patients who achieved viral clearance show enhanced viral clearance in the favipiravir group compared with the SOC group by day 29, with 72% of the favipiravir group and 52% of the SOC group being evaluable for viral clearance through day 29. The median time to viral clearance was 16.0 days (90% CI, 12.0 to 29.0) in the favipiravir group and 30.0 days (90% CI, 12.0 to 31.0) in the SOC group. A post hoc analysis revealed an effect in the subgroup of patients who were neutralizing antibody-negative at randomization. Treatment-emergent adverse events were equally distributed between the groups. Conclusions. We demonstrate that favipiravir can be safely administered to hospitalized adults with COVID-19 and believe that further studies are warranted. ClinicalTrials.gov registration. NCT04358549.
References
Bank, Renzette, Liu, An experimental evaluation of drug-induced mutational meltdown as an antiviral treatment strategy, Evolution
Cai, Yang, Liu, Experimental treatment with favipiravir for COVID-19: an open-label control study, Engineering
Cohen, Monoclonal antibodies to disrupt progression of early Covid-19 infection, N Engl J Med
Driouich, Cochin, Lingas, Favipiravir antiviral efficacy against SARS-CoV-2 in a hamster model, Nat Commun
Hung, Ghula, Aziz, The efficacy and adverse effects of favipiravir on COVID-19 patients: a systematic review and meta-analysis of published clinical trials and observational studies, Lancet, doi:10.2139/ssrn.3889346
Jensen, Lynch, Considering mutational meltdown as a potential SARS-CoV-2 treatment strategy, Heredity (Edinb)
Kaptein, Jacobs, Langendries, Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity, Proc Natl Acad Sci U S A
Naydenova, Muir, Wu, Structure of the SARS-CoV-2 RNAdependent RNA polymerase in the presence of favipiravir-RTP, Proc Natl Acad Sci U S A
Ormond, Liu, Matuszewski, The combined effect of oseltamivir and favipiravir on influenza a virus evolution, Genome Biol Evol
Peng, Peng, Yuan, Structural basis of SARS-CoV-2 polymerase inhibition by favipiravir, Innovation
Sada, Saraya, Ishii, Detailed molecular interactions of favipiravir with SARS-CoV-2, SARS-CoV, MERS-CoV, and influenza virus polymerases in silico, Microorganisms
Shinkai, Tsushima, Tanaka, Efficacy and safety of favipiravir in moderate COVID-19 pneumonia patients without oxygen therapy: a randomized, phase III clinical trial, Infect Dis Ther
Simonis, Theobald, Fätkenheuer, A comparative analysis of remdesivir and other repurposed antivirals against SARS-CoV-2, EMBO Mol Med
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res