Dose-dependent impact of tixagevimab–cilgavimab as primary prevention against SARS-CoV-2 in immunocompromised individuals
et al., Scientific Reports, doi:10.1038/s41598-025-02240-3, May 2025
42nd treatment shown to reduce risk in
May 2022, now with p = 0.0066 from 19 studies, recognized in 33 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
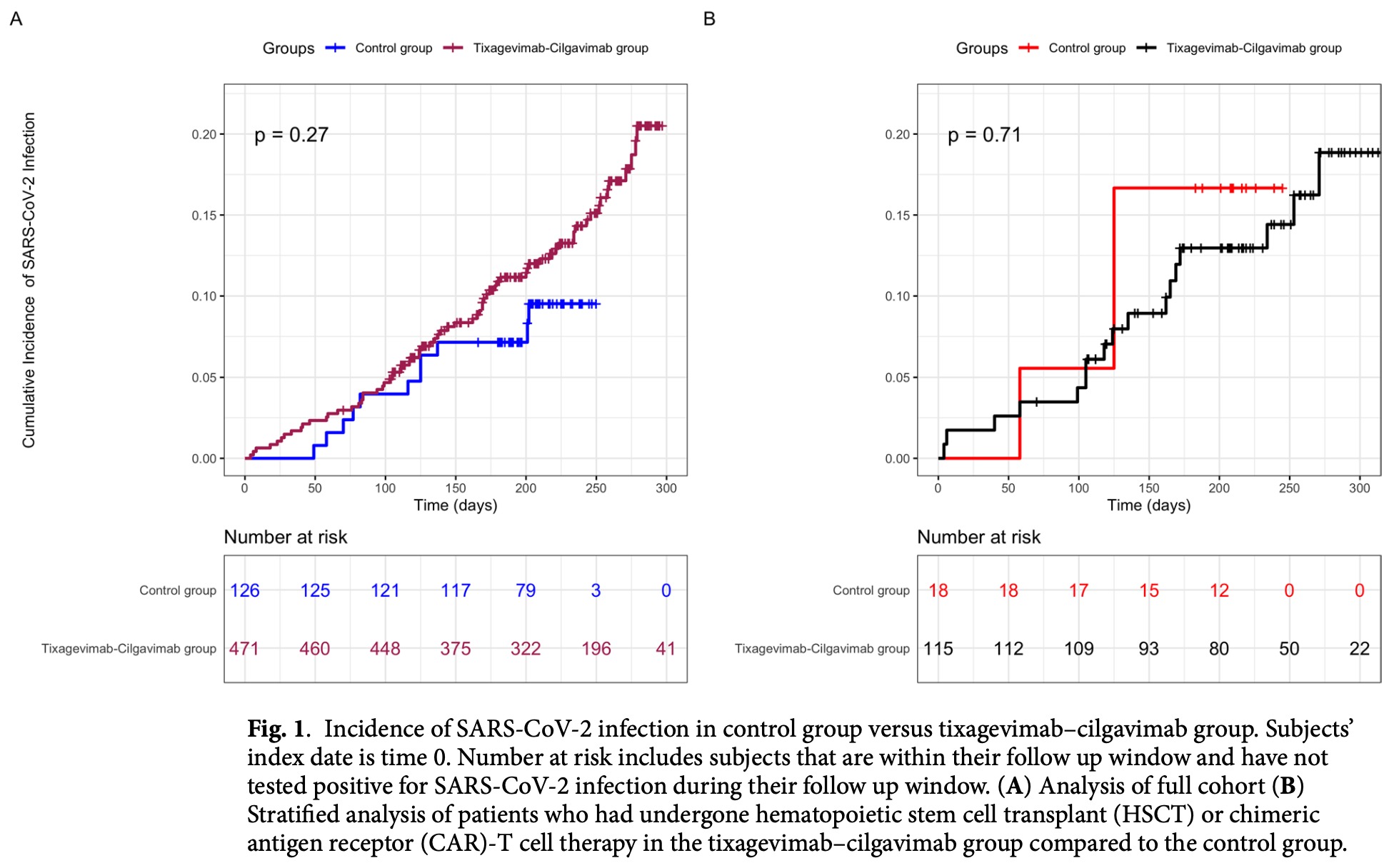
Retrospective 597 immunocompromised individuals showing no significant difference in infection rates over the entire study period. However, when truncating data to November 1, 2022 (before resistant variants dominated), effectiveness increased with higher cumulative doses.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2.75.2, BA.4.6, BQ.1.11, BA.5, BA.2.75, XBB2,3, XBB.1.53, ХВВ.1.9.13, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.14.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments5.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of case, 70.2% higher, RR 1.70, p = 0.08, treatment 70 of 471 (14.9%), control 11 of 126 (8.7%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Planas et al., Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies, bioRxiv, doi:10.1101/2022.11.17.516888.
2.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
3.
Uraki et al., Antiviral efficacy against and replicative fitness of an XBB.1.9.1 clinical isolate, iScience, doi:10.1016/j.isci.2023.108147.
Dluzynski et al., 21 May 2025, retrospective, USA, peer-reviewed, mean age 61.0, 9 authors, study period 5 January, 2022 - 14 December, 2022.
Contact: cpaules@pennstatehealth.psu.edu, cpaules23@gmail.com.
Dose-dependent impact of tixagevimab–cilgavimab as primary prevention against SARS-CoV-2 in immunocompromised individuals
Scientific Reports, doi:10.1038/s41598-025-02240-3
Tixagevimab-cilgavimab was available for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) prevention from December 2021 to January 2023, with dosing changes to reflect circulating variants. In a retrospective analysis of 597 immunocompromised individuals, incidence of SARS-CoV-2 infection was compared between those who did and did not receive tixagevimab-cilgavimab. A proportional hazards regression model with a time-dependent regressor for tixagevimab-cilgavimab dose was applied to assess cumulative doses. Secondary analyses were performed in hematopoietic stem cell transplant (HSCT) and chimeric antigen receptor (CAR)-T cell therapy recipients. There was no difference in SARS-CoV-2 infections between tixagevimab-cilgavimab recipients and controls (p = 0.27). There was a trend towards protection with increasing dose from 150 (HR 0.83, CI 0.50-1.38) to 600 mg (HR 0.48, CI 0.06-3.63) when truncating data on November 1st, 2022, which was also seen in HSCT or CAR-T cell therapy recipients, 150 mg (HR 0.71, CI 0.31-1.65) to 600 mg (HR 0.26, CI 0.01-7.47). This was most evident in immunocompromised individuals when variants neutralized by tixagevimab-cilgavimab in vitro were circulating; effectiveness 74%. Supports a proof of concept for monoclonal antibodies in immunocompromised individuals as a prevention strategy against novel viruses.
Author contributions D.D., P.S., C.H., S.S., N.M., J.S., M.H., V.C., and C.P. made substantial contributions to the conception and design of this study. D.D., P.S., C.H., S.S., N.M., and C.P. contributed to the data collection. D.D., P.S., and C.P. wrote the main manuscript. P.S. and V.C. processed the data and prepared the figures. All authors reviewed the manuscript.
Competing interests The authors declare no competing interests.
Additional information
Supplementary Information The online version contains supplementary material available at h t t p s : / / d o i . o r g / 1 0 . 1 0 3 8 / s 4 1 5 9 8 -0 2 5 -0 2 2 4 0 -3 . Correspondence and requests for materials should be addressed to C.I.P. Reprints and permissions information is available at www.nature.com/reprints . Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this..
References
Barnes, SARS-CoV-2-specific immune responses and clinical outcomes after COVID-19 vaccination in patients with immune-suppressive disease, Nat. Med, doi:10.1038/s41591-023-02414-4
Barouch, Covid-19 vaccines-Immunity, variants, boosters, N. Engl. J. Med, doi:10.1056/NEJMra2206573
Bruel, Longitudinal analysis of serum neutralization of SARS-CoV-2 Omicron BA.2, BA.4, and BA.5 in patients receiving monoclonal antibodies, Cell Rep. Med, doi:10.1016/j.xcrm.2022.100850
Bruel, Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies, Nat. Med, doi:10.1038/s41591-022-01792-5
Chen, Emerging dominant SARS-CoV-2 variants, J. Chem. Inf. Model, doi:10.1021/acs.jcim.2c01352
De Lamballerie, Low serum neutralization of Omicron variants a month after AZD7442 prophylaxis initiation, J. Infect, doi:10.1016/j.jinf.2022.10.006
Gisaid, SARS-CoV-2 sequences by variant, Dec 18, 2023
Hammitt, Nirsevimab for prevention of RSV in healthy late-preterm and term infants, N. Engl. J. Med, doi:10.1056/NEJMoa2110275
Harpaz, Dahl, Dooling, Prevalence of Immunosuppression among US Adults, JAMA, doi:10.1001/jama.2016.16477
Jondreville, Pre-exposure prophylaxis with tixagevimab/cilgavimab (AZD7442) prevents severe SARS-CoV-2 infection in recipients of allogeneic hematopoietic stem cell transplantation during the Omicron wave: A multicentric retrospective study of SFGM-TC, J. Hematol. Oncol, doi:10.1186/s13045-022-01387-0
Kurhade, Low neutralization of SARS-CoV-2 Omicron BA.275.2, BQ.1.1 and XBB.1 by parental mRNA vaccine or a BA.5 bivalent booster, Nat. Med, doi:10.1021/acs.jcim.2c01352
Laracy, Predictors of SARS-CoV-2 Omicron breakthrough infection after receipt of AZD7442 (tixagevimab-cilgavimab) for pre-exposure prophylaxis among hematologic malignancy patients, Haematologica, doi:10.3324/haematol.2023.283015
Lee, Efficacy of covid-19 vaccines in immunocompromised patients: Systematic review and meta-analysis, BMJ, doi:10.1136/bmj-2021-068632
Levin, Intramuscular AZD7442 (tixagevimab-cilgavimab) for prevention of covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2116620
Marovich, Mascola, Cohen, Monoclonal antibodies for prevention and treatment of COVID-19, JAMA, doi:10.1001/jama.2020.10245
Najjar-Debbiny, Gronich, Weber, Stein, Saliba, Effectiveness of evusheld in immunocompromised patients: Propensity score-matched analysis, Clin. Infect. Dis, doi:10.1093/cid/ciac855
Ocon, Mustafa, Real-world experience of tixagevimab and cilgavimab (evusheld) in rheumatologic patients on rituximab, J. Clin. Rheumatol, doi:10.1097/RHU.0000000000001907
Ocon, Real-world effectiveness of tixagevimab and cilgavimab (evusheld) in patients with hematological malignancies, J. Hematol, doi:10.14740/jh1062
Parker, Response to additional COVID-19 vaccine doses in people who are immunocompromised: A rapid review, Lancet Glob. Health, doi:10.1016/S2214-109X(21)00593-3
Planas, Bruel, Staropoli, Resistance of Omicron subvariants BA2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies, bioRxiv, doi:10.1101/2022.11.17.516888
Randi, COVID-19 in hematopoietic stem-cell transplant recipients: A systematic review and meta-analysis of clinical characteristics and outcomes, Rev. Med. Virol, doi:10.1002/rmv.2483
Sindu, Pre-exposure prophylaxis with tixagevimab-cilgavimab did not reduce severity of COVID-19 in lung transplant recipients with breakthrough infection, Transplant Direct, doi:10.1097/TXD.0000000000001485
Singson, Factors associated with severe outcomes among immunocompromised adults hospitalized for COVID-19-COVID-NET, 10 States, March 2020-February 2022, MMWR Morb. Mortal. Wkly Rep, doi:10.15585/mmwr.mm7127a3
Tong, Real-world efficacy and safety of tixagevimab plus cilgavimab in patients with cancer, J. Hematol. Oncol. Pharm
Touret, In vitro activity of therapeutic antibodies against SARS-CoV-2 Omicron BA.1, BA.2 and BA.5. Sci. Rep, doi:10.1038/s41598-022-16964-z
Tregoning, Flight, Higham, Wang, Pierce, Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape, Nat. Rev. Immunol, doi:10.1038/s41577-021-00592-1
Xhaard, Risk factors for a severe form of COVID-19 after allogeneic haematopoietic stem cell transplantation: A Société Francophone de Greffe de Moelle et de Thérapie cellulaire (SFGM-TC) multicentre cohort study, Br. J. Haematol, doi:10.1111/bjh.17260
Xue, Impact of tixagevimab/cilgavimab prophylaxis in patients undergoing allogeneic hematopoietic stem cell transplants and CAR T-cell therapy: A single center experience, Curr. Res. Transl. Med, doi:10.1016/j.retram.2023.103402
Young-Xu, Tixagevimab/cilgavimab for preventing COVID-19 during the Omicron surge: Retrospective analysis of National Veterans Health Administration electronic data, MBio, doi:10.1128/mbio.01024-23
Zhao, .7, BQ.1.1 and XBB.1.5 in individuals receiving Evusheld, J. Med. Virol, doi:10.1002/jmv.28932
DOI record:
{
"DOI": "10.1038/s41598-025-02240-3",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-025-02240-3",
"alternative-id": [
"2240"
],
"article-number": "17578",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "27 January 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "12 May 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "21 May 2025"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"ORCID": "https://orcid.org/0009-0000-6779-7788",
"affiliation": [],
"authenticated-orcid": false,
"family": "Dluzynski",
"given": "Daniela",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ssentongo",
"given": "Paddy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hale",
"given": "Cory M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shaheen",
"given": "Shareef K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maglakelidze",
"given": "Natella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sivik",
"given": "Jeffrey M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Henao",
"given": "Maria Paula",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chinchilli",
"given": "Vernon M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paules",
"given": "Catharine I.",
"sequence": "additional"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
5,
21
]
],
"date-time": "2025-05-21T04:48:25Z",
"timestamp": 1747802905000
},
"deposited": {
"date-parts": [
[
2025,
5,
21
]
],
"date-time": "2025-05-21T04:48:29Z",
"timestamp": 1747802909000
},
"indexed": {
"date-parts": [
[
2025,
5,
22
]
],
"date-time": "2025-05-22T04:07:07Z",
"timestamp": 1747886827514,
"version": "3.41.0"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2025,
5,
21
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2025,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
5,
21
]
],
"date-time": "2025-05-21T00:00:00Z",
"timestamp": 1747785600000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
5,
21
]
],
"date-time": "2025-05-21T00:00:00Z",
"timestamp": 1747785600000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-025-02240-3.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-025-02240-3",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-025-02240-3.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2025,
5,
21
]
]
},
"published-online": {
"date-parts": [
[
2025,
5,
21
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "2240_CR1",
"unstructured": "Data from: Provisional COVID-19 Deaths by Sex and Age. (Centers for Disease Control and Prevention, 2023)."
},
{
"key": "2240_CR2",
"unstructured": "WHO Coronavirus (COVID-19) Dashboard. World Health Organization. Accessed September 2nd, 2023. https://covid19.who.int/ (2023)"
},
{
"DOI": "10.1038/s41577-021-00592-1",
"author": "JS Tregoning",
"doi-asserted-by": "publisher",
"first-page": "626",
"issue": "10",
"journal-title": "Nat. Rev. Immunol.",
"key": "2240_CR3",
"unstructured": "Tregoning, J. S., Flight, K. E., Higham, S. L., Wang, Z. & Pierce, B. F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 21(10), 626–636. https://doi.org/10.1038/s41577-021-00592-1 (2021).",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.15585/mmwr.mm7127a3",
"author": "JRC Singson",
"doi-asserted-by": "publisher",
"first-page": "878",
"issue": "27",
"journal-title": "MMWR Morb. Mortal. Wkly Rep.",
"key": "2240_CR4",
"unstructured": "Singson, J. R. C. et al. Factors associated with severe outcomes among immunocompromised adults hospitalized for COVID-19-COVID-NET, 10 States, March 2020–February 2022. MMWR Morb. Mortal. Wkly Rep. 71(27), 878–884. https://doi.org/10.15585/mmwr.mm7127a3 (2022).",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1001/jama.2016.16477",
"author": "R Harpaz",
"doi-asserted-by": "publisher",
"first-page": "2547",
"issue": "23",
"journal-title": "JAMA",
"key": "2240_CR5",
"unstructured": "Harpaz, R., Dahl, R. M. & Dooling, K. L. Prevalence of Immunosuppression among US Adults, 2013. JAMA 316(23), 2547–2548. https://doi.org/10.1001/jama.2016.16477 (2016).",
"volume": "316",
"year": "2016"
},
{
"DOI": "10.1016/S2214-109X(21)00593-3",
"author": "EPK Parker",
"doi-asserted-by": "publisher",
"first-page": "e326",
"issue": "3",
"journal-title": "Lancet Glob. Health",
"key": "2240_CR6",
"unstructured": "Parker, E. P. K. et al. Response to additional COVID-19 vaccine doses in people who are immunocompromised: A rapid review. Lancet Glob. Health 10(3), e326–e328. https://doi.org/10.1016/S2214-109X(21)00593-3 (2022).",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1056/NEJMra2206573",
"author": "DH Barouch",
"doi-asserted-by": "publisher",
"first-page": "1011",
"issue": "11",
"journal-title": "N. Engl. J. Med.",
"key": "2240_CR7",
"unstructured": "Barouch, D. H. Covid-19 vaccines—Immunity, variants, boosters. N. Engl. J. Med. 387(11), 1011–1020. https://doi.org/10.1056/NEJMra2206573 (2022).",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1136/bmj-2021-068632",
"author": "ARYB Lee",
"doi-asserted-by": "publisher",
"first-page": "e068632",
"journal-title": "BMJ",
"key": "2240_CR8",
"unstructured": "Lee, A. R. Y. B. et al. Efficacy of covid-19 vaccines in immunocompromised patients: Systematic review and meta-analysis. BMJ 376, e068632. https://doi.org/10.1136/bmj-2021-068632 (2022).",
"volume": "376",
"year": "2022"
},
{
"DOI": "10.1038/s41591-023-02414-4",
"author": "E Barnes",
"doi-asserted-by": "publisher",
"first-page": "1760",
"issue": "7",
"journal-title": "Nat. Med.",
"key": "2240_CR9",
"unstructured": "Barnes, E. et al. SARS-CoV-2-specific immune responses and clinical outcomes after COVID-19 vaccination in patients with immune-suppressive disease. Nat. Med. 29(7), 1760–1774. https://doi.org/10.1038/s41591-023-02414-4 (2023).",
"volume": "29",
"year": "2023"
},
{
"key": "2240_CR10",
"unstructured": "EVUSHELD: Emergency Use Authorization (EAU)—Full fact sheet for healthcare providers. US Food and Drug Administration. Accessed 06 May 2022, https://www.fda.gov/media/154701/download"
},
{
"key": "2240_CR11",
"unstructured": "FDA announces Evusheld is not currently authorized for emergency use in the U.S. (U.S. Food and Drug Administration, 2023)."
},
{
"DOI": "10.1056/NEJMoa2116620",
"author": "MJ Levin",
"doi-asserted-by": "publisher",
"first-page": "2188",
"issue": "23",
"journal-title": "N. Engl. J. Med.",
"key": "2240_CR12",
"unstructured": "Levin, M. J. et al. Intramuscular AZD7442 (tixagevimab–cilgavimab) for prevention of covid-19. N. Engl. J. Med. 386(23), 2188–2200. https://doi.org/10.1056/NEJMoa2116620 (2022).",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1038/s41591-022-01792-5",
"author": "T Bruel",
"doi-asserted-by": "publisher",
"first-page": "1297",
"issue": "6",
"journal-title": "Nat. Med.",
"key": "2240_CR13",
"unstructured": "Bruel, T. et al. Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies. Nat. Med. 28(6), 1297–1302. https://doi.org/10.1038/s41591-022-01792-5 (2022).",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1038/s41598-022-16964-z",
"author": "F Touret",
"doi-asserted-by": "publisher",
"first-page": "12609",
"issue": "1",
"journal-title": "Sci. Rep.",
"key": "2240_CR14",
"unstructured": "Touret, F. et al. In vitro activity of therapeutic antibodies against SARS-CoV-2 Omicron BA.1, BA.2 and BA.5. Sci. Rep. 12(1), 12609. https://doi.org/10.1038/s41598-022-16964-z (2022).",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1002/rmv.2483",
"author": "BA Randi",
"doi-asserted-by": "publisher",
"issue": "6",
"journal-title": "Rev. Med. Virol.",
"key": "2240_CR15",
"unstructured": "Randi, B. A. et al. COVID-19 in hematopoietic stem-cell transplant recipients: A systematic review and meta-analysis of clinical characteristics and outcomes. Rev. Med. Virol. 33(6), e2483. https://doi.org/10.1002/rmv.2483 (2023).",
"volume": "33",
"year": "2023"
},
{
"DOI": "10.1186/s13045-022-01387-0",
"author": "L Jondreville",
"doi-asserted-by": "publisher",
"first-page": "169",
"issue": "1",
"journal-title": "J. Hematol. Oncol.",
"key": "2240_CR16",
"unstructured": "Jondreville, L. et al. Pre-exposure prophylaxis with tixagevimab/cilgavimab (AZD7442) prevents severe SARS-CoV-2 infection in recipients of allogeneic hematopoietic stem cell transplantation during the Omicron wave: A multicentric retrospective study of SFGM-TC. J. Hematol. Oncol. 15(1), 169. https://doi.org/10.1186/s13045-022-01387-0 (2022).",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1111/bjh.17260",
"author": "A Xhaard",
"doi-asserted-by": "publisher",
"first-page": "e121",
"issue": "5",
"journal-title": "Br. J. Haematol.",
"key": "2240_CR17",
"unstructured": "Xhaard, A. et al. Risk factors for a severe form of COVID-19 after allogeneic haematopoietic stem cell transplantation: A Société Francophone de Greffe de Moelle et de Thérapie cellulaire (SFGM-TC) multicentre cohort study. Br. J. Haematol. 192(5), e121–e124. https://doi.org/10.1111/bjh.17260 (2021).",
"volume": "192",
"year": "2021"
},
{
"DOI": "10.1016/j.retram.2023.103402",
"author": "E Xue",
"doi-asserted-by": "publisher",
"first-page": "103402",
"issue": "3",
"journal-title": "Curr. Res. Transl. Med.",
"key": "2240_CR18",
"unstructured": "Xue, E. et al. Impact of tixagevimab/cilgavimab prophylaxis in patients undergoing allogeneic hematopoietic stem cell transplants and CAR T-cell therapy: A single center experience. Curr. Res. Transl. Med. 71(3), 103402. https://doi.org/10.1016/j.retram.2023.103402 (2023).",
"volume": "71",
"year": "2023"
},
{
"key": "2240_CR19",
"unstructured": "GISAID. SARS-CoV-2 sequences by variant, Dec 18, 2023. Our World in Data. Accessed January 6, 2024. https://gisaid.org/"
},
{
"DOI": "10.1038/s41591-022-02162-x",
"author": "C Kurhade",
"doi-asserted-by": "publisher",
"first-page": "344",
"issue": "2",
"journal-title": "Nat. Med.",
"key": "2240_CR20",
"unstructured": "Kurhade, C. et al. Low neutralization of SARS-CoV-2 Omicron BA.275.2, BQ.1.1 and XBB.1 by parental mRNA vaccine or a BA.5 bivalent booster. Nat. Med. 29(2), 344–347. https://doi.org/10.1038/s41591-022-02162-x (2023).",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.1021/acs.jcim.2c01352",
"author": "J Chen",
"doi-asserted-by": "publisher",
"first-page": "335",
"issue": "1",
"journal-title": "J. Chem. Inf. Model.",
"key": "2240_CR21",
"unstructured": "Chen, J. et al. Emerging dominant SARS-CoV-2 variants. J. Chem. Inf. Model. 63(1), 335–342. https://doi.org/10.1021/acs.jcim.2c01352 (2023).",
"volume": "63",
"year": "2023"
},
{
"DOI": "10.1128/mbio.01024-23",
"author": "Y Young-Xu",
"doi-asserted-by": "publisher",
"first-page": "e0102423",
"issue": "4",
"journal-title": "MBio",
"key": "2240_CR22",
"unstructured": "Young-Xu, Y. et al. Tixagevimab/cilgavimab for preventing COVID-19 during the Omicron surge: Retrospective analysis of National Veterans Health Administration electronic data. MBio 14(4), e0102423. https://doi.org/10.1128/mbio.01024-23 (2023).",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac855",
"author": "R Najjar-Debbiny",
"doi-asserted-by": "publisher",
"first-page": "1067",
"issue": "6",
"journal-title": "Clin. Infect. Dis.",
"key": "2240_CR23",
"unstructured": "Najjar-Debbiny, R., Gronich, N., Weber, G., Stein, N. & Saliba, W. Effectiveness of evusheld in immunocompromised patients: Propensity score-matched analysis. Clin. Infect. Dis. 76(6), 1067–1073. https://doi.org/10.1093/cid/ciac855 (2023).",
"volume": "76",
"year": "2023"
},
{
"author": "A Tong",
"first-page": "302",
"issue": "6",
"journal-title": "J. Hematol. Oncol. Pharm.",
"key": "2240_CR24",
"unstructured": "Tong, A. et al. Real-world efficacy and safety of tixagevimab plus cilgavimab in patients with cancer. J. Hematol. Oncol. Pharm. 13(6), 302–312 (2023).",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1097/TXD.0000000000001485",
"author": "D Sindu",
"doi-asserted-by": "publisher",
"issue": "6",
"journal-title": "Transplant Direct.",
"key": "2240_CR25",
"unstructured": "Sindu, D. et al. Pre-exposure prophylaxis with tixagevimab–cilgavimab did not reduce severity of COVID-19 in lung transplant recipients with breakthrough infection. Transplant Direct. 9(6), e1485. https://doi.org/10.1097/TXD.0000000000001485 (2023).",
"volume": "9",
"year": "2023"
},
{
"DOI": "10.14740/jh1062",
"author": "AJ Ocon",
"doi-asserted-by": "publisher",
"first-page": "210",
"issue": "6",
"journal-title": "J. Hematol.",
"key": "2240_CR26",
"unstructured": "Ocon, A. J. et al. Real-world effectiveness of tixagevimab and cilgavimab (evusheld) in patients with hematological malignancies. J. Hematol. 11(6), 210–215. https://doi.org/10.14740/jh1062 (2022).",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1097/RHU.0000000000001907",
"author": "AJ Ocon",
"doi-asserted-by": "publisher",
"first-page": "109",
"issue": "2",
"journal-title": "J. Clin. Rheumatol.",
"key": "2240_CR27",
"unstructured": "Ocon, A. J. & Mustafa, S. S. Real-world experience of tixagevimab and cilgavimab (evusheld) in rheumatologic patients on rituximab. J. Clin. Rheumatol. 29(2), 109–111. https://doi.org/10.1097/RHU.0000000000001907 (2023).",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.1016/j.xcrm.2022.100850",
"author": "T Bruel",
"doi-asserted-by": "publisher",
"first-page": "100850",
"issue": "12",
"journal-title": "Cell Rep. Med.",
"key": "2240_CR28",
"unstructured": "Bruel, T. et al. Longitudinal analysis of serum neutralization of SARS-CoV-2 Omicron BA.2, BA.4, and BA.5 in patients receiving monoclonal antibodies. Cell Rep. Med. 3(12), 100850. https://doi.org/10.1016/j.xcrm.2022.100850 (2022).",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1101/2022.11.17.516888",
"doi-asserted-by": "publisher",
"key": "2240_CR29",
"unstructured": "Planas, D., Bruel, T., Staropoli, I, et al. Resistance of Omicron subvariants BA2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies. bioRxiv. https://doi.org/10.1101/2022.11.17.516888 (2022)."
},
{
"DOI": "10.1002/jmv.28932",
"author": "Q Zhao",
"doi-asserted-by": "publisher",
"first-page": "e28932",
"issue": "7",
"journal-title": "J. Med. Virol.",
"key": "2240_CR30",
"unstructured": "Zhao, Q. et al. Serum neutralization of SARS-CoV-2 Omicron BA.2, BA.2.75, BA.2.76, BA.5, BF.7, BQ.1.1 and XBB.1.5 in individuals receiving Evusheld. J. Med. Virol. 95(7), e28932. https://doi.org/10.1002/jmv.28932 (2023).",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1016/j.jinf.2022.10.006",
"author": "X de Lamballerie",
"doi-asserted-by": "publisher",
"first-page": "66",
"issue": "1",
"journal-title": "J. Infect.",
"key": "2240_CR31",
"unstructured": "de Lamballerie, X. et al. Low serum neutralization of Omicron variants a month after AZD7442 prophylaxis initiation. J. Infect. 86(1), 66–117. https://doi.org/10.1016/j.jinf.2022.10.006 (2023).",
"volume": "86",
"year": "2023"
},
{
"DOI": "10.3324/haematol.2023.283015",
"author": "JC Laracy",
"doi-asserted-by": "publisher",
"first-page": "3058",
"issue": "11",
"journal-title": "Haematologica",
"key": "2240_CR32",
"unstructured": "Laracy, J. C. et al. Predictors of SARS-CoV-2 Omicron breakthrough infection after receipt of AZD7442 (tixagevimab–cilgavimab) for pre-exposure prophylaxis among hematologic malignancy patients. Haematologica 108(11), 3058–3067. https://doi.org/10.3324/haematol.2023.283015 (2023).",
"volume": "108",
"year": "2023"
},
{
"DOI": "10.1001/jama.2020.10245",
"author": "M Marovich",
"doi-asserted-by": "publisher",
"first-page": "131",
"issue": "2",
"journal-title": "JAMA",
"key": "2240_CR33",
"unstructured": "Marovich, M., Mascola, J. R. & Cohen, M. S. Monoclonal antibodies for prevention and treatment of COVID-19. JAMA 324(2), 131–132. https://doi.org/10.1001/jama.2020.10245 (2020).",
"volume": "324",
"year": "2020"
},
{
"key": "2240_CR34",
"unstructured": "AstraZeneca reinforces commitment to protecting the most vulnerable from serious infectious diseases at IDWeek 2023. AstraZeneca; 2023. Accessed January 6, 2023. https://www.astrazeneca.com/media-centre/press-releases/2023/astrazeneca-reinforces-commitment-to-protecting-the-most-vulnerable-from-serious-infectious-diseases-at-idweek-2023.html"
},
{
"DOI": "10.1056/NEJMoa2110275",
"author": "LL Hammitt",
"doi-asserted-by": "publisher",
"first-page": "837",
"issue": "9",
"journal-title": "N. Engl. J. Med.",
"key": "2240_CR35",
"unstructured": "Hammitt, L. L. et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N. Engl. J. Med. 386(9), 837–846. https://doi.org/10.1056/NEJMoa2110275 (2022).",
"volume": "386",
"year": "2022"
},
{
"key": "2240_CR36",
"unstructured": "Administration USFaD. Fact Sheet for Healthcare Providers: Emergency Use Authorization of Pemgarda (Pemivibart) [Fact Sheet] (2024)."
}
],
"reference-count": 36,
"references-count": 36,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-025-02240-3"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Dose-dependent impact of tixagevimab–cilgavimab as primary prevention against SARS-CoV-2 in immunocompromised individuals",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "15"
}
