Pre-exposure Prophylaxis with Tixagevimab-cilgavimab did not Reduce Severity of COVID-19 in Lung Transplant Recipients with Breakthrough Infection
et al., Transplantation Direct, doi:10.1097/txd.0000000000001485, May 2023
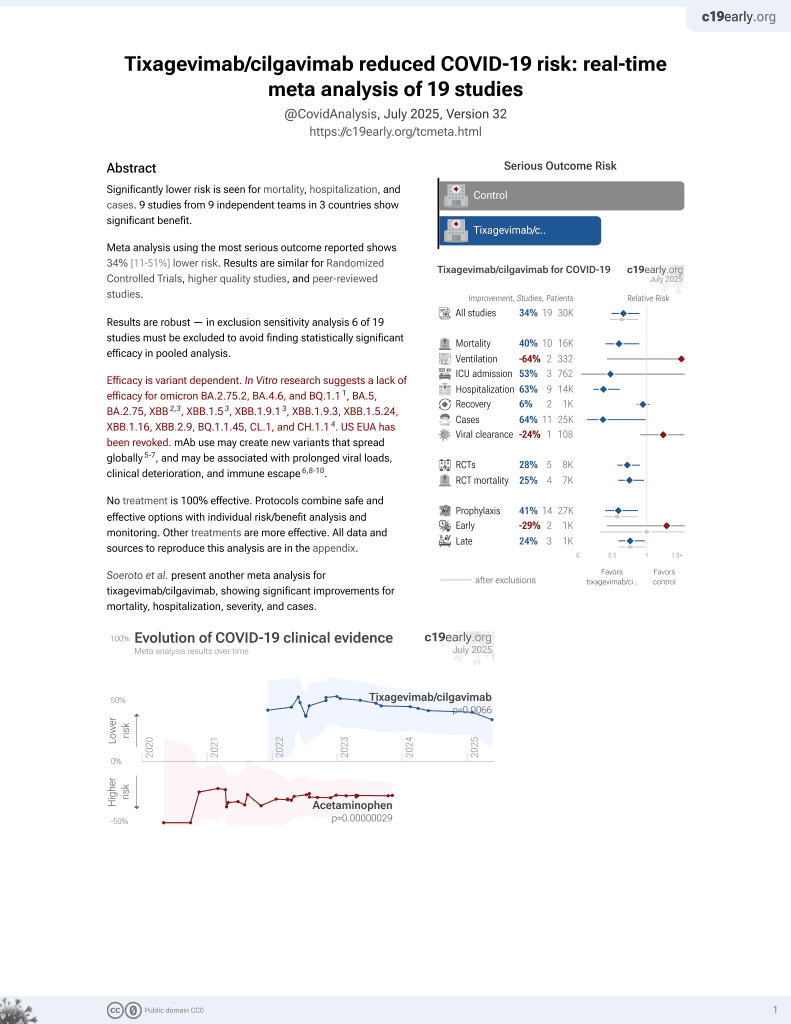
42nd treatment shown to reduce risk in
May 2022, now with p = 0.0066 from 19 studies, recognized in 33 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 546 lung transplant recipients, 203 receiving tixagevimab/cilgavimab, and 343 out of state or declining treatment, showing a trend towards lower incidence of cases, but no significant difference in clinical outcomes.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2.75.2, BA.4.6, BQ.1.11, BA.5, BA.2.75, XBB2,3, XBB.1.53, ХВВ.1.9.13, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.14.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments5.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 1.5% higher, HR 1.01, p = 0.99, treatment 2 of 17 (11.8%), control 2 of 17 (11.8%), propensity score matching, Cox proportional hazards.
|
|
risk of mechanical ventilation, 95.8% higher, HR 1.96, p = 0.58, propensity score matching, Cox proportional hazards.
|
|
risk of ICU admission, 209.6% higher, HR 3.10, p = 0.33, propensity score matching, Cox proportional hazards.
|
|
risk of hospitalization, 53.2% lower, HR 0.47, p = 0.17, propensity score matching, Cox proportional hazards.
|
|
risk of symptomatic case, 28.9% lower, RR 0.71, p = 0.14, treatment 24 of 203 (11.8%), control 57 of 343 (16.6%), NNT 21, unadjusted.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Planas et al., Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies, bioRxiv, doi:10.1101/2022.11.17.516888.
2.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
3.
Uraki et al., Antiviral efficacy against and replicative fitness of an XBB.1.9.1 clinical isolate, iScience, doi:10.1016/j.isci.2023.108147.
Sindu et al., 12 May 2023, retrospective, USA, peer-reviewed, median age 67.4, 7 authors, study period December 2021 - August 2022.
Contact: sofya.tokman@dignityhealth.org.
Pre-exposure Prophylaxis with Tixagevimab-cilgavimab did not Reduce Severity of COVID-19 in Lung Transplant Recipients with Breakthrough Infection
Transplantation Direct, doi:10.1097/txd.0000000000001485
I nfection with the severe acute respiratory syndrome coro- navirus 2 (SARS-CoV-2) and the resulting COVID-19 have led to significant morbidity and mortality among solid organ transplant recipients since the start of the pandemic. 1 Mutations in the viral spike protein have led to the emergence of numerous variants, with the Omicron variant and its sublineages dominating since December 2021. 2, 3 Although the rates of hospitalization and in-hospital mortality fell in the general population during the Omicron surge, the morbidity and mortality among solid organ transplant recipients remained disproportionately high. 4, 5 Vaccination is the cornerstone of prevention of severe COVID-19 in the general population; however, the immune response to vaccination among solid organ transplant recipients is often inadequate, leaving them susceptible to severe illness. A study of 658 solid organ transplant recipients, including 71 lung transplant recipients (LTRs), showed that only 39% of LTRs developed an antibody response after 2 doses of a SARS-CoV-2 mRNA vaccine. 6 Furthermore, although a third vaccine dose improved the humoral response
Lung Transplantation Background. Lung transplant recipients (LTRs) have an increased risk of COVID-19-related morbidity and mortality. Tixagevimab-cilgavimab (tix-cil) is a long-acting monoclonal antibody combination granted Emergency Use Authorization approval by the US Food and Drug Administration for COVID-19 pre-exposure prophylaxis (PrEP) in immunocompromised patients. We sought to determine whether tix-cil 300-300 mg reduced the incidence and disease severity of severe acute respiratory syndrome coronavirus 2 infection in LTRs during the Omicron wave. Methods. We performed a retrospective, single-center cohort study of LTRs who had received a COVID-19 diagnosis between December 2021 and August 2022. We compared baseline characteristics and clinical outcomes after COVID-19 between LTRs who received tix-cil PrEP and those who did not. We then conducted propensity-score matching based on baseline characteristics and therapeutic interventions and compared clinical outcomes between the 2 groups. Results. Of 203 LTRs who received tix-cil PrEP and 343 who did not, 24 (11.8%) and 57 (16.6%), respectively, developed symptomatic COVID-19 (hazard ratio [HR], 0.669; 95% confidence interval [CI], 0.415-1.079; P = 0.099). The hospitalization rate of LTRs with COVID-19 during the Omicron wave trended lower in the tix-cil group than in the non-tix-cil group (20.8% versus 43.1%; HR, 0.430; 95% CI, 0.165-1.118; P = 0.083). In propensity-matched analyses, 17 LTRs who received tix-cil and 17 LTRs who did not had similar rates of hospitalization (HR, 0.468;
References
Adjei, Hong, Molinari, Mortality risk among patients hospitalized primarily for COVID-19 during the Omicron and Delta variant pandemic periods -United States, MMWR Morb Mortal Wkly Rep
Austin, An introduction to propensity score methods for reducing the effects of confounding in observational studies, Multivariate Behav Res
Benotmane, Gautier, Perrin, Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses, JAMA
Benotmane, Velay, Gautier-Vargas, Breakthrough COVID-19 cases despite prophylaxis with 150 mg of tixagevimab and 150 mg of cilgavimab in kidney transplant recipients, Am J Transplant
Boyarsky, Werbel, Avery, Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients, JAMA
Bruel, Hadjadj, Maes, Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies, Nat Med
Centers, Services, COVID-19 monoclonal antibodies
Dauriat, Beaumont, Nguyen, Efficacy of three COVID-19 vaccine doses in lung transplant recipients: a multicentre cohort study, Eur Respir J
Fisher, Schlauch, Mulloy, Outcomes of COVID-19 in hospitalized solid organ transplant recipients compared to a matched cohort of non-transplant patients at a national healthcare system in the United States, Clin Transplant
Focosi, Casadevall, A critical analysis of the use of cilgavimab plus tixagevimab monoclonal antibody cocktail (Evusheld™) for COVID-19 prophylaxis and treatment, Viruses
Heldman, Kates, Safa, UW COVID-19 SOT Study Team. COVID-19 in hospitalized lung and non-lung solid organ transplant recipients: a comparative analysis from a multicenter study, Am J Transplant
Iketani, Liu, Guo, Antibody evasion properties of SARS-CoV-2 Omicron sublineages, Nature
Keam, Tixagevimab + cilgavimab: first approval, Drugs
Kertes, Shapiro, David, Engel-Zohar, Association between AZD7442 (tixagevimab-cilgavimab) administration and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, hospitalization, and mortality, Clin Infect Dis
Kneidinger, Hecker, Bessa, Outcome of lung transplant recipients infected with SARS-CoV-2/Omicron/B.1.1.529: a nationwide German study, Infection. 2022 Sept
Lakens, Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs, Front Psychol
Levin, Ustianowski, Wit, Intramuscular AZD7442 (tixagevimab-cilgavimab) for prevention of Covid-19, N Engl J Med
Meng, Abdullahi, Ferreira, Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity, Nature
Messika, Eloy, Roux, COVID-19 in lung transplant recipients, Transplantation
Najjar-Debbiny, Gronich, Weber, Effectiveness of Evusheld in immunocompromised patients: propensity score-matched analysis, Clin Infect Dis
Nguyen, Flahault, Chavarot, AP-HP-Centre Monoclonal Antibodies Working Group. Pre-exposure prophylaxis with tixagevimab and cilgavimab (Evusheld) for COVID-19 among 1112 severely immunocompromised patients, Clin Microbiol Infect
Ordaya, Beam, Yao, Characterization of early-onset severe acute respiratory syndrome coronavirus 2 infection in immunocompromised patients who received tixagevimab-cilgavimab prophylaxis, Open Forum Infect Dis
Pereira, Mohan, Cohen, COVID-19 in solid organ transplant recipients: initial report from the US epicenter, Am J Transplant
Saez-Giménez, Berastegui, Barrecheguren, COVID-19 in lung transplant recipients: a multicenter study, Am J Transplant
Vanblargan, Errico, Halfmann, An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies, Nat Med
Zost, Gilchuk, Case, Potently neutralizing and protective human antibodies against SARS-CoV-2, Nature
DOI record:
{
"DOI": "10.1097/txd.0000000000001485",
"ISSN": [
"2373-8731"
],
"URL": "http://dx.doi.org/10.1097/txd.0000000000001485",
"author": [
{
"affiliation": [],
"family": "Sindu",
"given": "Devika",
"sequence": "first"
},
{
"affiliation": [],
"family": "Razia",
"given": "Deepika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grief",
"given": "Katherine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cherrier",
"given": "Lauren",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Omar",
"given": "Ashraf",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Walia",
"given": "Rajat",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tokman",
"given": "Sofya",
"sequence": "additional"
}
],
"container-title": "Transplantation Direct",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
5,
15
]
],
"date-time": "2023-05-15T16:47:35Z",
"timestamp": 1684169255000
},
"deposited": {
"date-parts": [
[
2023,
5,
15
]
],
"date-time": "2023-05-15T16:47:54Z",
"timestamp": 1684169274000
},
"indexed": {
"date-parts": [
[
2023,
5,
16
]
],
"date-time": "2023-05-16T04:47:59Z",
"timestamp": 1684212479496
},
"is-referenced-by-count": 0,
"issue": "6",
"issued": {
"date-parts": [
[
2023,
5,
12
]
]
},
"journal-issue": {
"issue": "6",
"published-print": {
"date-parts": [
[
2023
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://journals.lww.com/10.1097/TXD.0000000000001485",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "276",
"original-title": [],
"page": "e1485",
"prefix": "10.1097",
"published": {
"date-parts": [
[
2023,
5,
12
]
]
},
"published-online": {
"date-parts": [
[
2023,
5,
12
]
]
},
"publisher": "Ovid Technologies (Wolters Kluwer Health)",
"reference": [
{
"DOI": "10.1097/TP.0000000000003508",
"article-title": "COVID-19 in lung transplant recipients.",
"author": "Messika",
"doi-asserted-by": "crossref",
"first-page": "177",
"journal-title": "Transplantation",
"key": "R1-20230515",
"volume": "105",
"year": "2021"
},
{
"DOI": "10.1038/s41586-022-04474-x",
"article-title": "Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity.",
"author": "Meng",
"doi-asserted-by": "crossref",
"first-page": "706",
"journal-title": "Nature",
"key": "R3-20230515",
"volume": "603",
"year": "2022"
},
{
"DOI": "10.15585/mmwr.mm7137a4",
"article-title": "Mortality risk among patients hospitalized primarily for COVID-19 during the Omicron and Delta variant pandemic periods - United States, April 2020-June 2022.",
"author": "Adjei",
"doi-asserted-by": "crossref",
"first-page": "1182",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "R4-20230515",
"volume": "71",
"year": "2022"
},
{
"article-title": "Outcome of lung transplant recipients infected with SARS-CoV-2/Omicron/B.1.1.529: a nationwide German study.",
"author": "Kneidinger",
"first-page": "1",
"journal-title": "Infection",
"key": "R5-20230515",
"year": "2022 Sept 9"
},
{
"DOI": "10.1001/jama.2021.7489",
"article-title": "Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients.",
"author": "Boyarsky",
"doi-asserted-by": "crossref",
"first-page": "2204",
"journal-title": "JAMA",
"key": "R6-20230515",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.12339",
"article-title": "Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses.",
"author": "Benotmane",
"doi-asserted-by": "crossref",
"first-page": "1063",
"journal-title": "JAMA",
"key": "R7-20230515",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1183/13993003.00502-2022",
"article-title": "Efficacy of three COVID-19 vaccine doses in lung transplant recipients: a multicentre cohort study.",
"author": "Dauriat",
"doi-asserted-by": "crossref",
"first-page": "2200502",
"journal-title": "Eur Respir J",
"key": "R8-20230515",
"volume": "61",
"year": "2023"
},
{
"DOI": "10.1038/s41591-021-01678-y",
"article-title": "An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies.",
"author": "VanBlargan",
"doi-asserted-by": "crossref",
"first-page": "490",
"journal-title": "Nat Med",
"key": "R9-20230515",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1038/s41586-020-2548-6",
"article-title": "Potently neutralizing and protective human antibodies against SARS-CoV-2.",
"author": "Zost",
"doi-asserted-by": "crossref",
"first-page": "443",
"journal-title": "Nature",
"key": "R10-20230515",
"volume": "584",
"year": "2020"
},
{
"DOI": "10.1007/s40265-022-01731-1",
"article-title": "Tixagevimab + cilgavimab: first approval.",
"author": "Keam",
"doi-asserted-by": "crossref",
"first-page": "1001",
"journal-title": "Drugs",
"key": "R12-20230515",
"volume": "82",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04594-4",
"article-title": "Antibody evasion properties of SARS-CoV-2 Omicron sublineages.",
"author": "Iketani",
"doi-asserted-by": "crossref",
"first-page": "553",
"journal-title": "Nature",
"key": "R14-20230515",
"volume": "604",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116620",
"article-title": "Intramuscular AZD7442 (tixagevimab-cilgavimab) for prevention of Covid-19.",
"author": "Levin",
"doi-asserted-by": "crossref",
"first-page": "2188",
"journal-title": "N Engl J Med",
"key": "R15-20230515",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1080/00273171.2011.568786",
"article-title": "An introduction to propensity score methods for reducing the effects of confounding in observational studies.",
"author": "Austin",
"doi-asserted-by": "crossref",
"first-page": "399",
"journal-title": "Multivariate Behav Res",
"key": "R16-20230515",
"volume": "46",
"year": "2011"
},
{
"DOI": "10.3389/fpsyg.2013.00863",
"article-title": "Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs.",
"author": "Lakens",
"doi-asserted-by": "crossref",
"first-page": "863",
"journal-title": "Front Psychol",
"key": "R18-20230515",
"volume": "4",
"year": "2013"
},
{
"DOI": "10.1111/ctr.14216",
"article-title": "Outcomes of COVID-19 in hospitalized solid organ transplant recipients compared to a matched cohort of non-transplant patients at a national healthcare system in the United States.",
"author": "Fisher",
"doi-asserted-by": "crossref",
"first-page": "e14216",
"journal-title": "Clin Transplant",
"key": "R19-20230515",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1111/ajt.16692",
"article-title": "COVID-19 in hospitalized lung and non-lung solid organ transplant recipients: a comparative analysis from a multicenter study.",
"author": "Heldman",
"doi-asserted-by": "crossref",
"first-page": "2774",
"journal-title": "Am J Transplant",
"key": "R20-20230515",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1111/ajt.15941",
"article-title": "COVID-19 in solid organ transplant recipients: initial report from the US epicenter.",
"author": "Pereira",
"doi-asserted-by": "crossref",
"first-page": "1800",
"journal-title": "Am J Transplant",
"key": "R21-20230515",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1111/ajt.16364",
"article-title": "COVID-19 in lung transplant recipients: a multicenter study.",
"author": "Saez-Giménez",
"doi-asserted-by": "crossref",
"first-page": "1816",
"journal-title": "Am J Transplant",
"key": "R22-20230515",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciac855",
"article-title": "Effectiveness of Evusheld in immunocompromised patients: propensity score-matched analysis.",
"author": "Najjar-Debbiny",
"doi-asserted-by": "crossref",
"first-page": "1067",
"journal-title": "Clin Infect Dis",
"key": "R23-20230515",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac625",
"article-title": "Association between AZD7442 (tixagevimab-cilgavimab) administration and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, hospitalization, and mortality.",
"author": "Kertes",
"doi-asserted-by": "crossref",
"first-page": "e126",
"journal-title": "Clin Infect Dis",
"key": "R24-20230515",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1016/j.cmi.2022.07.015",
"article-title": "Pre-exposure prophylaxis with tixagevimab and cilgavimab (Evusheld) for COVID-19 among 1112 severely immunocompromised patients.",
"author": "Nguyen",
"doi-asserted-by": "crossref",
"first-page": "1654.e1",
"journal-title": "Clin Microbiol Infect",
"key": "R25-20230515",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1093/ofid/ofac283",
"article-title": "Characterization of early-onset severe acute respiratory syndrome coronavirus 2 infection in immunocompromised patients who received tixagevimab-cilgavimab prophylaxis.",
"author": "Ordaya",
"doi-asserted-by": "crossref",
"first-page": "ofac283",
"journal-title": "Open Forum Infect Dis",
"key": "R26-20230515",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1111/ajt.17121",
"article-title": "Breakthrough COVID-19 cases despite prophylaxis with 150 mg of tixagevimab and 150 mg of cilgavimab in kidney transplant recipients.",
"author": "Benotmane",
"doi-asserted-by": "crossref",
"first-page": "2675",
"journal-title": "Am J Transplant",
"key": "R27-20230515",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.3390/v14091999",
"article-title": "A critical analysis of the use of cilgavimab plus tixagevimab monoclonal antibody cocktail (Evusheld™) for COVID-19 prophylaxis and treatment.",
"author": "Focosi",
"doi-asserted-by": "crossref",
"first-page": "1999",
"journal-title": "Viruses",
"key": "R28-20230515",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1038/s41591-022-01792-5",
"article-title": "Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies.",
"author": "Bruel",
"doi-asserted-by": "crossref",
"first-page": "1297",
"journal-title": "Nat Med",
"key": "R29-20230515",
"volume": "28",
"year": "2022"
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.lww.com/10.1097/TXD.0000000000001485"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Transplantation"
],
"subtitle": [],
"title": "Pre-exposure Prophylaxis with Tixagevimab-cilgavimab did not Reduce Severity of COVID-19 in Lung Transplant Recipients with Breakthrough Infection",
"type": "journal-article",
"volume": "9"
}