Effectiveness of Evusheld in Immunocompromised Patients: Propensity Score-Matched Analysis
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciac855, Oct 2022
42nd treatment shown to reduce risk in
May 2022, now with p = 0.0066 from 19 studies, recognized in 33 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 732 immunocompromised patients in Israel treated with tixagevimab/cilgavimab, and 2,812 matched controls, showing significantly lower cases and hospitalization with treatment.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2.75.2, BA.4.6, BQ.1.11, BA.5, BA.2.75, XBB2,3, XBB.1.53, ХВВ.1.9.13, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.14.
|
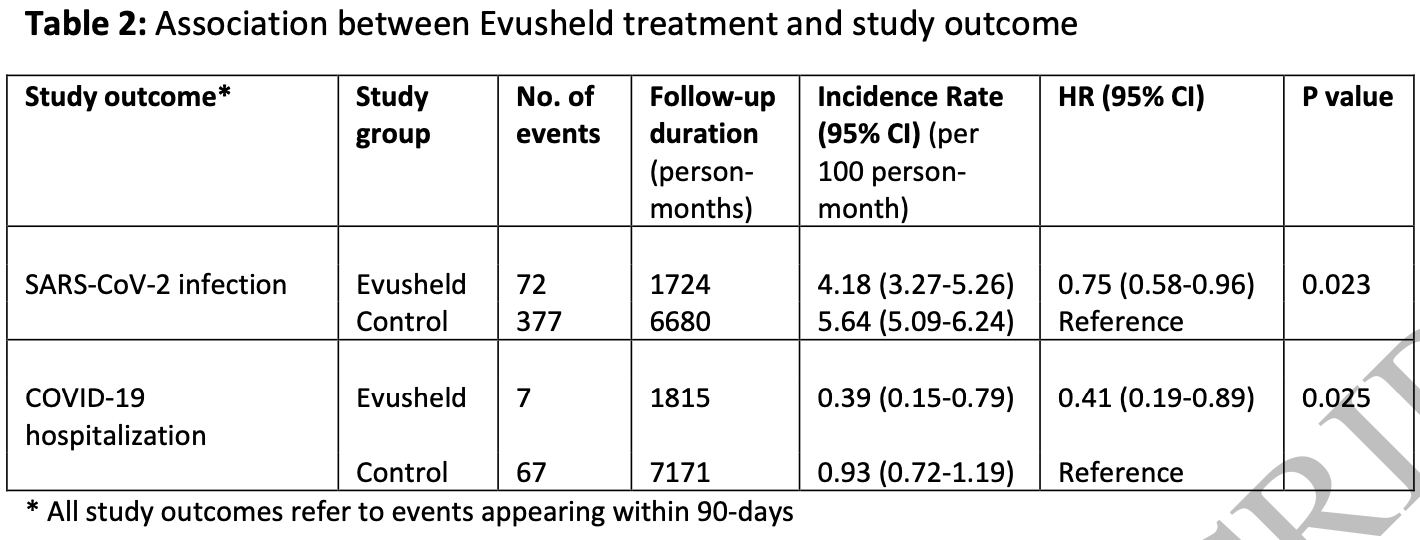
risk of hospitalization, 59.0% lower, HR 0.41, p = 0.02, treatment 72 of 703 (10.2%), control 377 of 2,812 (13.4%), Cox proportional hazards.
|
|
risk of case, 25.0% lower, HR 0.75, p = 0.03, treatment 72 of 703 (10.2%), control 377 of 2,812 (13.4%), NNT 32, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Planas et al., Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies, bioRxiv, doi:10.1101/2022.11.17.516888.
2.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Najjar-Debbiny et al., 31 Oct 2022, retrospective, Israel, peer-reviewed, 5 authors.
Contact: ronzana@clalit.org.il, ronza.najjar@gmail.com.
Effectiveness of Evusheld in Immunocompromised Patients: Propensity Score-Matched Analysis
Background: Tixagevimab and Cilgavimab, a combined monoclonal antibody (Evusheld) was granted emergency use authorization for SARS-CoV-2 preexposure prophylaxis in individuals with moderate to severe immunocompromising condition. In this study we used population-based real-world data to evaluate the effectiveness of Evusheld in immunocompromised patients. Methods: Using the computerized database of the largest healthcare provider in Israel, we identified all adult immunocompromised patients who were eligible to receive Evusheld (the dose used during the study period was 150mg Tixagevimab and 150mg Cilgavimab) on 15-February-2022. Patients with a documentation of a prior SARS-CoV-2 infection were excluded. A total of 703 patients who received Evusheld were propensity score-matched, using a ratio of 1:4, with 2812 patients who have not received Evusheld (control group). Patients were followed through 30-June-2022 for up to 90-days for the first documentation of SARS-CoV-2 infection and COVID-19 related hospitalization. Results: Overall, 72 patients in the Evusheld group and 377 patients in the control group had SARS-CoV-2 infection, reflecting and incidence rate of 4.18 and 5.64 per 100 person-months, respectively. HR was 0.75(95%CI,0.58-0.96) for SARS-CoV-2 infection, and 0.41(0.19-0.89) for COVID-19 related hospitalization in the Evusheld group compared to the control group. The magnitude of relative risks reduction of each outcome was greater in non-obese patients (P for interaction=0.020 and 0.045, respectively).
Conclusion: This study suggests that Evusheld (150mg Tixagevimab and 150mg Cilgavimab) is effective in reducing the risk of SARS-CoV-2 infection and COVID-19 hospitalization in immunocompromised patients. The effectiveness of this dose appears to be greater in non-obese patients
Despite that, information about study outcomes and the administration of COVID-19 vaccines, collected prospectively as part of the Israeli MOH COVID-19 database, are considered complete. Information about Evusheld is also closely monitored by CHS and is considered complete. In addition, this retrospective cohort study is observational in nature, hence albeit using propensity score-matched analysis to adjust for confounders, residual confounding remains of concern. Furthermore, undocumented positive home antigen tests and prior asymptomatic SARS-CoV-2 infection is still a concern. However, we assume this might have happened in a minority of immunocompromised patients, a population with a high awareness of their vulnerable health status, and since COVID-19 follow-up services and eligibility for COVID-19 treatments, which might be crucial for these patients, required a documented test. Moreover, SARS-CoV-2 PCR and institutional antigen tests (performed by a healthcare worker and documented) were highly available and free of charge throughout the country. Finally, the outcome measured in the study was laboratory confirmed COVID-19 infection by means of positive PCR or antigen test regardless of symptoms.
Conclusions This study suggests that Evusheld is effective in reducing COVID-19 infections and related hospitalizations in immunocompromised adult patients regardless of their vaccination status. Adjusted weight dosing approach is suggested but needs to be further studied.
..
References
-Austin, An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies, Multivariate Behav Res, doi:10.1080/00273171.2011.568786
-Kertes, David, Engel-Zohar, Rosen, Hemo et al., Association between AZD7442 (tixagevimab-cilgavimab) administration and SARS-CoV-2 infection, hospitalization and mortality, Clin Infect Dis, doi:10.1093/cid/ciac625
-Lee, Wong, Chai, Lee, Lee et al., Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis, BMJ, doi:10.1136/bmj-2021-068632
-Levin, Ustianowski, Wit, Launay, Avila et al., Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for Prevention of Covid-19, N Engl J Med, doi:10.1056/NEJMoa2116620
-Najjar-Debbiny, Gronich, Weber, Khoury, Amar et al., Effectiveness of Paxlovid in Reducing Severe COVID-19 and Mortality in High Risk Patients, Clin Infect Dis, doi:10.1093/cid/ciac443
-Touret, Baronti, Bouzidi, De Lamballerie, In vitro evaluation of therapeutic antibodies against a SARS-CoV-2 Omicron B.1.1.529 isolate, Sci Rep, doi:10.1038/s41598-022-08559-5
-Yanir, Doweck, Shibli, Najjar-Debbiny, Saliba, Association Between the BNT162b2 Messenger RNA COVID-19 Vaccine and the Risk of Sudden Sensorineural Hearing Loss, JAMA Otolaryngol Head Neck Surg, doi:10.1001/jamaoto.2021.4278
Furer, Eviatar, Zisman, Peleg, Paran et al., Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study, Ann Rheum Dis, doi:10.1136/annrheumdis-2021-220647
Montgomery, Hobbs, Padilla, Arbetter, Templeton et al., Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial, Lancet Respir Med, doi:10.1016/S2213-2600(22)00180-1
DOI record:
{
"DOI": "10.1093/cid/ciac855",
"ISSN": [
"1058-4838",
"1537-6591"
],
"URL": "http://dx.doi.org/10.1093/cid/ciac855",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Tixagevimab and Cilgavimab, a combined monoclonal antibody (Evusheld) was granted emergency use authorization for SARS-CoV-2 preexposure prophylaxis in individuals with moderate to severe immunocompromising condition. In this study we used population-based real-world data to evaluate the effectiveness of Evusheld in immunocompromised patients.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>Using the computerized database of the largest healthcare provider in Israel, we identified all adult immunocompromised patients who were eligible to receive Evusheld (the dose used during the study period was 150 mg Tixagevimab and 150 mg Cilgavimab) on 15-February-2022. Patients with a documentation of a prior SARS-CoV-2 infection were excluded. A total of 703 patients who received Evusheld were propensity score-matched, using a ratio of 1:4, with 2812 patients who have not received Evusheld (control group). Patients were followed through 30-June-2022 for up to 90-days for the first documentation of SARS-CoV-2 infection and COVID-19 related hospitalization.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Overall, 72 patients in the Evusheld group and 377 patients in the control group had SARS-CoV-2 infection, reflecting and incidence rate of 4.18 and 5.64 per 100 person-months, respectively. HR was 0.75(95%CI,0.58-0.96) for SARS-CoV-2 infection, and 0.41(0.19-0.89) for COVID-19 related hospitalization in the Evusheld group compared to the control group. The magnitude of relative risks reduction of each outcome was greater in non-obese patients (P for interaction = 0.020 and 0.045, respectively).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>This study suggests that Evusheld (150 mg Tixagevimab and 150 mg Cilgavimab) is effective in reducing the risk of SARS-CoV-2 infection and COVID-19 hospitalization in immunocompromised patients. The effectiveness of this dose appears to be greater in non-obese patients</jats:p>\n </jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0001-7586-3714",
"affiliation": [
{
"name": "Infection Control and Prevention Unit, Lady Davis Carmel Medical Center , Haifa , Israel"
},
{
"name": "Ruth and Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology , Haifa , Israel"
}
],
"authenticated-orcid": false,
"family": "Najjar-Debbiny",
"given": "Ronza",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Ruth and Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology , Haifa , Israel"
},
{
"name": "Department of Community Medicine and Epidemiology, Lady Davis Carmel Medical Center , Haifa , Israel"
}
],
"family": "Gronich",
"given": "Naomi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ruth and Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology , Haifa , Israel"
},
{
"name": "Infectious Diseases unit, Lady Davis Carmel Medical Center , Haifa , Israel"
}
],
"family": "Weber",
"given": "Gabriel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Community Medicine and Epidemiology, Lady Davis Carmel Medical Center , Haifa , Israel"
},
{
"name": "Statistical Unit, Lady Davis Carmel Medical Center , Haifa , Israel"
}
],
"family": "Stein",
"given": "Nili",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ruth and Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology , Haifa , Israel"
},
{
"name": "Department of Community Medicine and Epidemiology, Lady Davis Carmel Medical Center , Haifa , Israel"
},
{
"name": "Translational Epidemiology Unit and Research Authority, Lady Davis Carmel Medical Center , Haifa , Israel"
}
],
"family": "Saliba",
"given": "Walid",
"sequence": "additional"
}
],
"container-title": "Clinical Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
10,
31
]
],
"date-time": "2022-10-31T08:00:58Z",
"timestamp": 1667203258000
},
"deposited": {
"date-parts": [
[
2022,
10,
31
]
],
"date-time": "2022-10-31T08:00:59Z",
"timestamp": 1667203259000
},
"indexed": {
"date-parts": [
[
2022,
11,
1
]
],
"date-time": "2022-11-01T04:54:55Z",
"timestamp": 1667278495680
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
10,
31
]
]
},
"language": "en",
"license": [
{
"URL": "https://academic.oup.com/pages/standard-publication-reuse-rights",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
31
]
],
"date-time": "2022-10-31T00:00:00Z",
"timestamp": 1667174400000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciac855/46690004/ciac855.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciac855/46690004/ciac855.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022,
10,
31
]
]
},
"published-online": {
"date-parts": [
[
2022,
10,
31
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciac855/6780937"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Effectiveness of Evusheld in Immunocompromised Patients: Propensity Score-Matched Analysis",
"type": "journal-article"
}
