Clinical Effects of Streptococcus salivarius K12 in Hospitalized COVID-19 Patients: Results of a Preliminary Study
et al., Microorganisms, doi:10.3390/microorganisms10101926, NCT05043376, Sep 2022
Probiotics for COVID-19
20th treatment shown to reduce risk in
March 2021, now with p = 0.00000044 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
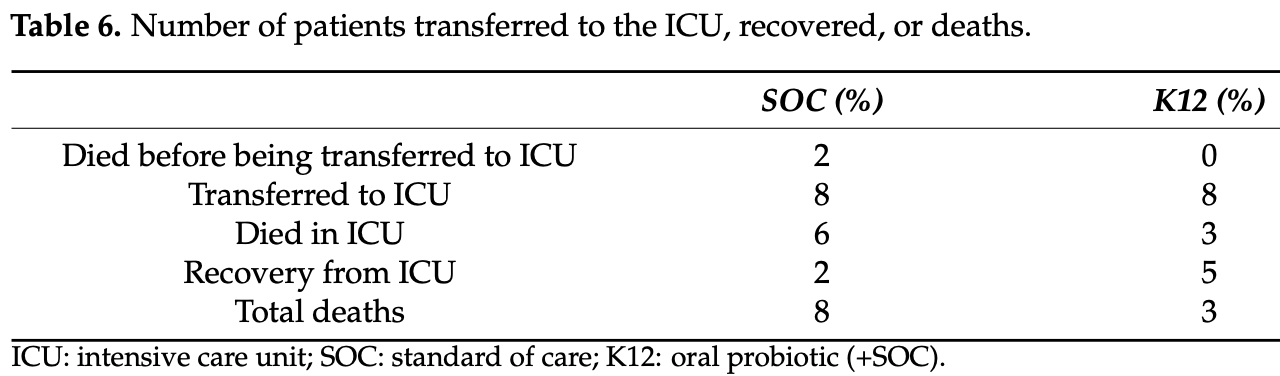
RCT 50 hospitalized patients in Pakistan, 25 treated with S. salivarius K12, showing lower mortality with treatment, without statistical significance. There were more patients with higher oxygen requirements at baseline in the control group - 18 vs. 6 with O2 ≥ 8 L/min.
Probiotic efficacy depends on the specific strains used. Specific microbes may decrease or increase COVID-19 risk1.
This study is excluded in the after exclusion results of meta-analysis:
unadjusted differences between groups.
|
risk of death, 62.5% lower, RR 0.38, p = 0.17, treatment 3 of 25 (12.0%), control 8 of 25 (32.0%), NNT 5.0.
|
|
risk of ICU admission, no change, RR 1.00, p = 1.00, treatment 8 of 25 (32.0%), control 8 of 25 (32.0%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Di Pierro et al., 28 Sep 2022, Randomized Controlled Trial, Pakistan, peer-reviewed, mean age 48.5, 7 authors, study period 11 August, 2021 - 18 November, 2021, trial NCT05043376 (history).
Contact: f.dipierro@vellejaresearch.com (corresponding author).
Clinical Effects of Streptococcus salivarius K12 in Hospitalized COVID-19 Patients: Results of a Preliminary Study
Microorganisms, doi:10.3390/microorganisms10101926
Anatomical and physiological considerations indicate that the oral cavity is a primary source of the lung microbiota community, and recent studies have shown that the microbiota in the lungs contributes to immunological homeostasis, potentially altering the organ's susceptibility to viral infection, including SARS-CoV-2. It has been proposed that, in the case of viral infection, lung Gram-negative bacteria could promote the cytokine cascade with a better performance than a microbiota mainly constituted by Gram-positive bacteria. Recent observations also suggest that Prevotella-rich oral microbiotas would dominate the oral cavity of SARS-CoV-2-infected patients. In comparison, Streptococcus-rich microbiotas would dominate the oral cavity of healthy people. To verify if the modulation of the oral microbiota could have an impact on the current coronavirus disease, we administered for 14 days a well-recognized and oral-colonizing probiotic (S. salivarius K12) to hospitalized COVID-19 patients. The preliminary results of our randomized and controlled trial seem to prove the potential role of this oral strain in improving the course of the main markers of pathology, as well as its ability to apparently reduce the death rate from COVID-19. Although in a preliminary and only circumstantial way, our results seem to confirm the hypothesis of a direct involvement of the oral microbiota in the construction of a lung microbiota whose taxonomic structure could modulate the inflammatory processes generated at the pulmonary and systemic level by a viral infection.
References
Adam, Streptococcus salivarius K12 and M18 Probiotics Reduce Periodontal Pathogen-Induced Inflammation
Akata, Van Eeden, Lung Macrophage Functional Properties in Chronic Obstructive Pulmonary Disease, Int. J. Mol. Sci, doi:10.3390/ijms21030853
Bao, Zhang, Dong, Zhao, Li et al., Oral Microbiome and SARS-CoV-2: Beware of Lung Co-infection, Front. Microbiol, doi:10.3389/fmicb.2020.01840
Bouwer, Saunderson, Dunn, Lester, Crowley et al., Rapid Interferon-Gamma Release from Natural Killer Cells Induced by a Streptococcal Commensal, J. Interf. Cytokine Res, doi:10.1089/jir.2012.0116
Bradley, Zeamer, Bucci, Cincotta, Salive et al., Oropharyngeal Microbiome Profiled at Admission is Predictive of the Need for Respiratory Support Among COVID-19 Patients, Med. Rxiv, doi:10.1101/2022.02.28.22271627
Burton, Chilcott, Wescombe, Tagg, Extended Safety Data for the Oral Cavity Probiotic Streptococcus salivarius K12, Probiotics Antimicrob. Proteins, doi:10.1007/s12602-010-9045-4
Burton, Cowley, Simon, Mckinney, Wescombe et al., Evaluation of safety and human tolerance of the oral probiotic Streptococcus salivarius K12: A randomized, placebo-controlled, double-blind study, Food Chem. Toxicol, doi:10.1016/j.fct.2011.06.038
Burton, Wescombe, Moore, Chilcott, Tagg, Safety Assessment of the Oral Cavity Probiotic Streptococcus salivarius K12, Appl. Environ. Microbiol, doi:10.1128/AEM.72.4.3050-3053.2006
Cernioglo, Kalanetra, Meier, Lewis, Underwood et al., Multi-Strain Probiotic Supplementation with a Product Containing Human-Native S. salivarius K12 in Healthy Adults Increases Oral S. salivarius, Nutrients, doi:10.3390/nu13124392
Channappanavar, Perlman, Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology, Semin. Immunopathol, doi:10.1007/s00281-017-0629-x
Delahooke, Barclay, Poxton, A re-appraisal of the biological activity of bacteroides LPS, J. Med. Microbiol, doi:10.1099/00222615-42-2-102
Di Pierro, A possible probiotic (S. salivarius K12) approach to improve oral and lung microbiotas and raise defenses against SAR S-CoV-2, Minerva Medica, doi:10.23736/S0026-4806.20.06570-2
Di Pierro, Adami, Rapacioli, Giardini, Streitberger, Clinical evaluation of the oral probiotic Streptococcus salivarius K12 in the prevention of recurrent pharyngitis and/or tonsillitis caused by Streptococcus pyogenes in adults, Expert Opin. Biol. Ther, doi:10.1517/14712598.2013.758711
Di Pierro, Colombo, Giuliani, Danza, Basile et al., Effect of administration of Streptococcus salivarius K12 on the occurrence of streptococcal pharyngo-tonsillitis, scarlet fever and acute otitis media in 3 years old children, Eur. Rev. Med. Pharmacol. Sci
Di Pierro, Colombo, The administration of S. salivarius K12 to children may reduce the rate of SARS-CoV-2 infection, Minerva Med, doi:10.23736/S0026-4806.21.07487-5
Di Pierro, Colombo, Zanvit, Risso, Rottoli, Use of Streptococcus salivarius K12 in the prevention of streptococcal and viral pharyngotonsillitis in children, Drug Health Patient Saf, doi:10.2147/DHPS.S59665
Di Pierro, Colombo, Zanvit, Rottoli, Positive clinical outcomes derived from using Streptococcus salivarius K12 to prevent streptococcal pharyngotonsillitis in children: A pilot investigation, Drug Health Patient Saf, doi:10.2147/DHPS.S117214
Di Pierro, Di Pasquale, Di Cicco, Oral use of Streptococcus salivarius K12 in children with secretory otitis media: Preliminary results of a pilot, uncontrolled study, Int. J. Gen. Med, doi:10.2147/IJGM.S92488
Di Pierro, Risso, Poggi, Timitilli, Bolloli et al., Use of Streptococcus salivarius K12 to reduce the incidence of pharyngo-tonsillitis and acute otitis media in children: A retrospective analysis in not-recurrent pediatric subjects, Minerva Pediatr, doi:10.23736/S0026-4946.18.05182-4
Filkins, Hampton, Gifford, Gross, Hogan et al., Prevalence of Streptococci and Increased Polymicrobial Diversity Associated with Cystic Fibrosis Patient Stability, J. Bacteriol, doi:10.1128/JB.00566-12
Haran, Bradley, Zeamer, Cincotta, Salive et al., Inflammation-type dysbiosis of the oral microbiome associates with the duration of COVID-19 symptoms and long COVID, JCI Insight, doi:10.1172/jci.insight.152346
Henry, De Oliveira, Benoit, Plebani, Lippi, Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis, Clin. Chem. Lab. Med, doi:10.1515/cclm-2020-0369
Hilty, Burke, Pedro, Cardenas, Bush et al., Disordered microbial communities in asthmatic airways, PLoS ONE, doi:10.1371/journal.pone.0008578
Hoogerwerf, De Vos, Bresser, Van Der Zee, Pater et al., Lung Inflammation Induced by Lipoteichoic Acid or Lipopolysaccharide in Humans, Am. J. Respir. Crit. Care Med, doi:10.1164/rccm.200708-1261OC
Huffnagle, Dickson, Lukacs, The respiratory tract microbiome and lung inflammation: A two-way street, Mucosal Immunol, doi:10.1038/mi.2016.108
Hyink, Wescombe, Upton, Ragland, Burton et al., Salivaricin A2 and the Novel Lantibiotic Salivaricin B Are Encoded at Adjacent Loci on a 190-Kilobase Transmissible Megaplasmid in the Oral Probiotic Strain Streptococcus salivarius K12, Appl. Environ. Microbiol, doi:10.1128/AEM.02265-06
Iebba, Zanotta, Campisciano, Zerbato, Di Bella et al., Profiling of Oral Microbiota and Cytokines in COVID-19 Patients, Front. Microbiol, doi:10.3389/fmicb.2021.671813
Jia, Zhi, Lai, Wang, Xia et al., The oral microbiota-A mechanistic role for systemic diseases, Br. Dent. J, doi:10.1038/sj.bdj.2018.217
Khan, Khan, COVID-2019-associated overexpressed Prevotella proteins mediated host-pathogen interactions and their role in coronavirus outbreak, Bioinformatics, doi:10.1093/bioinformatics/btaa285
Laws, Hale, Kemp, Human Systemic Immune Response to Ingestion of the Oral Probiotic Streptococcus salivarius BLIS K12, Probiotics Antimicrob. Proteins, doi:10.1007/s12602-021-09822-3
Li, Yang, Zhou, Disoma, Dong et al., Microbiome Profiling Using Shotgun Metagenomic Sequencing Identified Unique Microorganisms in COVID-19 Patients With Altered Gut Microbiota, Front. Microbiol, doi:10.3389/fmicb.2021.712081
Liu, Liu, Zhang, Lee, Wu et al., Association between the nasopharyngeal microbiome and metabolome in patients with COVID-19, Synth. Syst. Biotechnol, doi:10.1016/j.synbio.2021.06.002
Macdonald, Chanyi, Macklaim, Cadieux, Reid et al., Streptococcus salivarius inhibits immune activation by periodontal disease pathogens, BMC Oral Health, doi:10.1186/s12903-021-01606-z
Mammen, Scannapieco, Sethi, Oral-lung microbiome interactions in lung diseases, Periodontology
Marini, Sitzia, Panatta, De Vincentiis, Pilot study to explore the prophylactic efficacy of oral probiotic Streptococcus salivarius K12 in preventing recurrent pharyngo-tonsillar episodes in pediatric patients, Int. J. Gen. Med, doi:10.2147/IJGM.S168209
Miller, Annavajhala, Chong, Park, Nobel et al., Oral Microbiome Alterations and SARS-CoV-2 Saliva Viral Load in Patients with COVID-19, Microbiol. Spectr, doi:10.1128/Spectrum.00055-21
Moffatt, Cookson, The lung microbiome in health and disease, Clin. Med. (Lond.), doi:10.7861/clinmedicine.17-6-525
Mokhtar, Rismayuddin, Yassim, Ahmad, Wahab et al., Streptococcus salivarius K12 inhibits Candida albicans aggregation, biofilm formation and dimorphism, Biofouling, doi:10.1080/08927014.2021.1967334
Rosas-Salazar, Kimura, Shilts, Strickland, Freeman et al., SARS-CoV-2 infection and viral load are associated with the upper respiratory tract microbiome, J. Allergy Clin. Immunol, doi:10.1016/j.jaci.2021.02.001
Shen, Xiao, Kang, Ma, Shi et al., Genomic Diversity of Severe Acute Respiratory Syndrome-Coronavirus 2 in Patients With Coronavirus Disease, Clin. Infect. Dis, doi:10.1093/cid/ciaa203
Tamanai-Shacoori, Gall-David, Moussouni, Sweidan, Polard et al., SARS-CoV-2 and Prevotella spp.: Friend or foe? A systematic literature review, J. Med. Microbiol, doi:10.1099/jmm.0.001520
Ventero, Cuadrat, Vidal, Andrade, Molina-Pardines et al., Nasopharyngeal Microbial Communities of Patients Infected With SARS-CoV-2 That Developed COVID-19, Front. Microbiol, doi:10.3389/fmicb.2021.637430
Wang, Li, Sun, Gao, Wei et al., Bacterial colonization dampens influenza-mediated acute lung injury via induction of M2 alveolar macrophages, Nat. Commun, doi:10.1038/ncomms3106
Wang, Li, Tian, Role of microbiota on lung homeostasis and diseases, Sci. China Life Sci, doi:10.1007/s11427-017-9151-1
Wang, Lin, Xiang, Liu, Fang et al., Oropharyngeal Probiotic ENT-K12 Prevents Respiratory Tract Infections among Frontline Medical Staff Fighting Against COVID-19: A Pilot Study, Front. Bioeng. Biotechnol, doi:10.3389/fbioe.2021.646184
Wescombe, Hale, Heng, Tagg, Developing oral probiotics from Streptococcus salivarius, Future Microbiol, doi:10.2217/fmb.12.113
Wilcox, Stuart, Leaver, Lown, Willcox et al., Effectiveness of the probiotic Streptococcus salivarius K12 for the treatment and/or prevention of sore throat: A systematic review, Clin. Microbiol. Infect, doi:10.1016/j.cmi.2018.12.031
Yadava, Pattaroni, Sichelstiel, Trompette, Gollwitzer et al., Microbiota Promotes Chronic Pulmonary Inflammation by Enhancing IL-17A and Autoantibodies, Am. J. Respir. Crit. Care Med, doi:10.1164/rccm.201504-0779OC
Yu, Gail, Consonni, Carugno, Humphrys et al., Characterizing human lung tissue microbiota and its relationship to epidemiological and clinical features, Genome Biol, doi:10.1186/s13059-016-1021-1
Zemanick, Wagner, Robertson, Ahrens, Chmiel et al., Airway microbiota across age and disease spectrum in cystic fibrosis, Eur. Respir. J, doi:10.1183/13993003.00832-2017
DOI record:
{
"DOI": "10.3390/microorganisms10101926",
"ISSN": [
"2076-2607"
],
"URL": "http://dx.doi.org/10.3390/microorganisms10101926",
"abstract": "<jats:p>Anatomical and physiological considerations indicate that the oral cavity is a primary source of the lung microbiota community, and recent studies have shown that the microbiota in the lungs contributes to immunological homeostasis, potentially altering the organ’s susceptibility to viral infection, including SARS-CoV-2. It has been proposed that, in the case of viral infection, lung Gram-negative bacteria could promote the cytokine cascade with a better performance than a microbiota mainly constituted by Gram-positive bacteria. Recent observations also suggest that Prevotella-rich oral microbiotas would dominate the oral cavity of SARS-CoV-2-infected patients. In comparison, Streptococcus-rich microbiotas would dominate the oral cavity of healthy people. To verify if the modulation of the oral microbiota could have an impact on the current coronavirus disease, we administered for 14 days a well-recognized and oral-colonizing probiotic (S. salivarius K12) to hospitalized COVID-19 patients. The preliminary results of our randomized and controlled trial seem to prove the potential role of this oral strain in improving the course of the main markers of pathology, as well as its ability to apparently reduce the death rate from COVID-19. Although in a preliminary and only circumstantial way, our results seem to confirm the hypothesis of a direct involvement of the oral microbiota in the construction of a lung microbiota whose taxonomic structure could modulate the inflammatory processes generated at the pulmonary and systemic level by a viral infection.</jats:p>",
"alternative-id": [
"microorganisms10101926"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-6654-8675",
"affiliation": [],
"authenticated-orcid": false,
"family": "Di Pierro",
"given": "Francesco",
"sequence": "first"
},
{
"affiliation": [],
"family": "Iqtadar",
"given": "Somia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mumtaz",
"given": "Sami Ullah",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3922-9115",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bertuccioli",
"given": "Alexander",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Recchia",
"given": "Martino",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zerbinati",
"given": "Nicola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khan",
"given": "Amjad",
"sequence": "additional"
}
],
"container-title": "Microorganisms",
"container-title-short": "Microorganisms",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
9,
29
]
],
"date-time": "2022-09-29T02:53:19Z",
"timestamp": 1664419999000
},
"deposited": {
"date-parts": [
[
2022,
9,
29
]
],
"date-time": "2022-09-29T03:33:58Z",
"timestamp": 1664422438000
},
"indexed": {
"date-parts": [
[
2022,
9,
29
]
],
"date-time": "2022-09-29T04:57:54Z",
"timestamp": 1664427474372
},
"is-referenced-by-count": 0,
"issue": "10",
"issued": {
"date-parts": [
[
2022,
9,
28
]
]
},
"journal-issue": {
"issue": "10",
"published-online": {
"date-parts": [
[
2022,
10
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
28
]
],
"date-time": "2022-09-28T00:00:00Z",
"timestamp": 1664323200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2076-2607/10/10/1926/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1926",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
9,
28
]
]
},
"published-online": {
"date-parts": [
[
2022,
9,
28
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1007/s11427-017-9151-1",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.1093/cid/ciaa203",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.1183/13993003.00832-2017",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1371/journal.pone.0008578",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1038/sj.bdj.2018.217",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1038/ncomms3106",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1164/rccm.200708-1261OC",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.3390/ijms21030853",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1101/2022.02.28.22271627",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1093/bioinformatics/btaa285",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1016/j.jaci.2021.02.001",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.1172/jci.insight.152346",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1016/j.synbio.2021.06.002",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.3389/fmicb.2021.637430",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1128/Spectrum.00055-21",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1099/jmm.0.001520",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.3389/fmicb.2021.671813",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.23736/S0026-4806.20.06570-2",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.3390/nu13124392",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.3389/fmicb.2021.712081",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1128/JB.00566-12",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.3389/fmicb.2020.01840",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.23736/S0026-4806.21.07487-5",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.3389/fbioe.2021.646184",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1016/j.cmi.2018.12.031",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1007/s12602-010-9045-4",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1016/j.fct.2011.06.038",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.1128/AEM.72.4.3050-3053.2006",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.7861/clinmedicine.17-6-525",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.1111/prd.12301",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.1038/mi.2016.108",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1164/rccm.201504-0779OC",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1186/s13059-016-1021-1",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.1099/00222615-42-2-102",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.23736/S0026-4946.18.05182-4",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"article-title": "Effect of administration of Streptococcus salivarius K12 on the occurrence of streptococcal pharyngo-tonsillitis, scarlet fever and acute otitis media in 3 years old children",
"author": "Di Pierro",
"first-page": "4601",
"journal-title": "Eur. Rev. Med. Pharmacol. Sci.",
"key": "ref36",
"volume": "20",
"year": "2016"
},
{
"DOI": "10.2147/DHPS.S117214",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.2147/DHPS.S59665",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.2147/IJGM.S92488",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.1517/14712598.2013.758711",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.2147/IJGM.S168209",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1128/AEM.02265-06",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.1080/08927014.2021.1967334",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"DOI": "10.1186/s12903-021-01606-z",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"key": "ref45",
"unstructured": "Streptococcus salivarius K12 and M18 Probiotics Reduce Periodontal Pathogen-Induced Inflammation. Meeting. 2011; IADR/AADR/CADR General Session (San Diego, California)\nhttps://www.researchgate.net/publication/266764016_Streptococcus_salivarius_K12_and_M18_Probiotics_Reduce_Periodontal_Pathogen-induced_Inflammation"
},
{
"DOI": "10.1007/s00281-017-0629-x",
"doi-asserted-by": "publisher",
"key": "ref46"
},
{
"DOI": "10.1089/jir.2012.0116",
"doi-asserted-by": "publisher",
"key": "ref47"
},
{
"DOI": "10.2217/fmb.12.113",
"doi-asserted-by": "publisher",
"key": "ref48"
},
{
"DOI": "10.1007/s12602-021-09822-3",
"doi-asserted-by": "publisher",
"key": "ref49"
},
{
"DOI": "10.1515/cclm-2020-0369",
"doi-asserted-by": "publisher",
"key": "ref50"
}
],
"reference-count": 50,
"references-count": 50,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2076-2607/10/10/1926"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Virology",
"Microbiology (medical)",
"Microbiology"
],
"subtitle": [],
"title": "Clinical Effects of Streptococcus salivarius K12 in Hospitalized COVID-19 Patients: Results of a Preliminary Study",
"type": "journal-article",
"volume": "10"
}
