Proxalutamide (GT0918) Reduces the Rate of Hospitalization in mild-to-moderate COVID-19 Female Patients: A Randomized Double-Blinded Placebo-Controlled Two-Arm Parallel Trial
et al., medRxiv, doi:10.1101/2021.07.06.21260086, Jul 2021
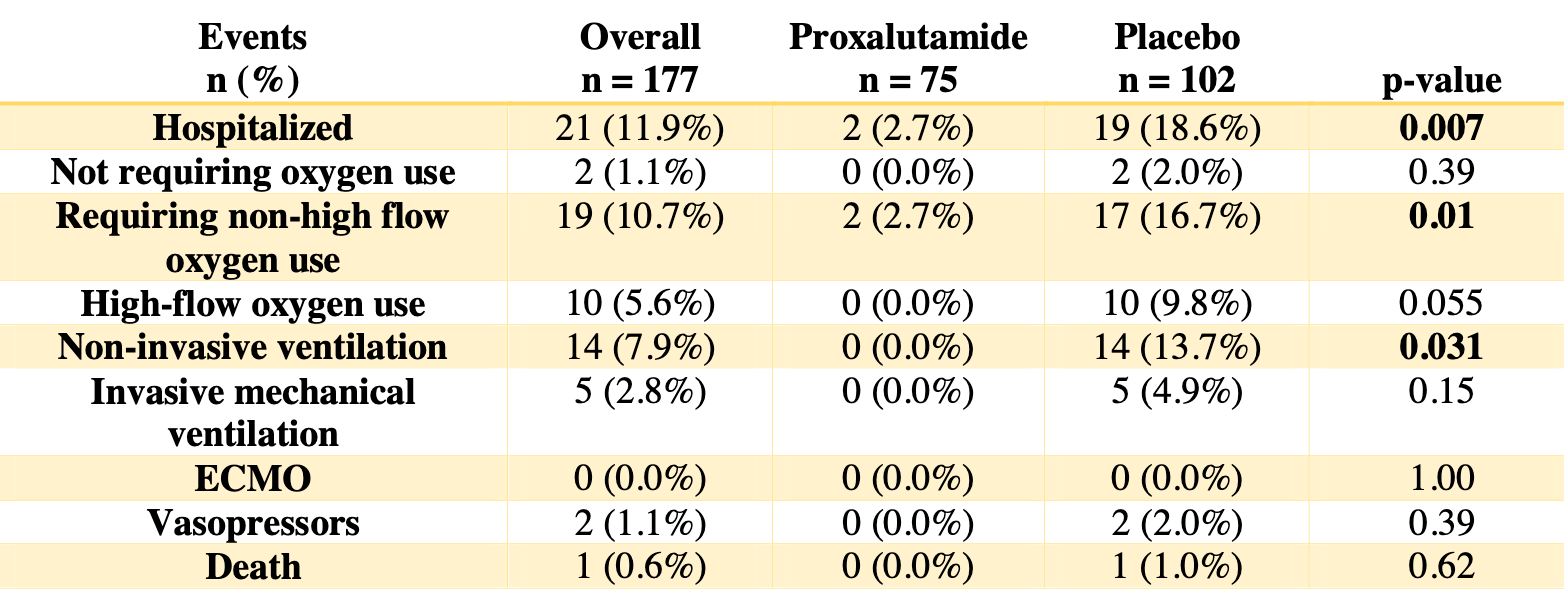
RCT 177 women in Brazil, 75 treated with proxalutamide, showing significantly lower hospitalization with treatment.
|
risk of death, 63.4% lower, RR 0.37, p = 1.00, treatment 0 of 75 (0.0%), control 1 of 102 (1.0%), NNT 102, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of mechanical ventilation, 89.7% lower, RR 0.10, p = 0.07, treatment 0 of 75 (0.0%), control 5 of 102 (4.9%), NNT 20, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of hospitalization, 85.7% lower, RR 0.14, p < 0.001, treatment 2 of 75 (2.7%), control 19 of 102 (18.6%), NNT 6.3.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Cadegiani et al., 10 Jul 2021, Double Blind Randomized Controlled Trial, Brazil, preprint, 7 authors, study period 4 January, 2021 - 28 February, 2021.
Proxalutamide (GT0918) Reduces the Rate of Hospitalization in mild-to-moderate COVID-19 Female Patients: A Randomized Double-Blinded Placebo-Controlled Two-Arm Parallel Trial
doi:10.1101/2021.07.06.21260086
Background: Antiandrogens were shown to be effective in mild-tomoderate COVID-19 male patients, supported by the SARS-CoV-2 dependency on transmembrane protease, serine 2 (TMPRSS2), which is solely modulated by androgens, for cell entry. While women with hyperandrogenism experiment more symptoms in COVID-19 compared to women without hyperandrogenism, and the chronic use of an antiandrogen seemed to mitigate these symptoms, whether the benefits would be observed in overall females is unknown. The objective of this study is to evaluate the efficacy of proxalutamide as a treatment for mild-to-moderate COVID-19 in females. Methods: This was a randomized, double-blind, placebo-controlled, two-arm, parallel study on COVID-19 female outpatients, that compared the use of proxalutamide versus placebo. The primary outcome was hospitalization rates throughout 30 days after randomization. Patients with laboratory confirmed COVID-19 not hospitalized were recruited in two sites in Brasilia, Brazil, between January 4 and February 28, 2021, were randomized on a 2:3 ratio between proxalutamide and placebo, and were administered proxalutamide 200mg/day or placebo for seven days, in addition to usual care. Results: A total of 177 women were randomized, including 75 in the proxalutamide arm and 102 patients in the placebo arm. None of the patients lost follow-up or discontinued treatment. The 30-day hospitalization rate was 2.7% in the proxalutamide arm and 18.6% in the placebo arm (p<0.001), with a hospitalization risk ratio (RR) of 0.14 [95% confidence interval (CI), 0.03-0.59]. Conclusions: These findings suggest that treatment of COVID-19 patients with proxalutamide in combination with standard of care was reduced hospitalization rate by 86% (p < 0.001), with no safety concerns. (Clinicaltrials.gov: NCT04853134)
References
Cadegiani, Lim, Goren, Mccoy, Situm et al., Clinical symptoms of hyperandrogenic women diagnosed with COVID-19, J Eur Acad Dermatol Venereol, doi:10.1111/jdv.17004
Cadegiani, Mccoy, Wambier, Goren, Early Antiandrogen Therapy With Dutasteride Reduces Viral Shedding, Inflammatory Responses, and Time-to-Remission in Males With COVID-19: A Randomized, Double-Blind, Placebo-Controlled Interventional Trial (EAT-DUTA AndroCoV Trial -Biochemical), Cureus, doi:10.7759/cureus.13047
Cadegiani, Repurposing existing drugs for COVID-19: an endocrinology perspective, BMC Endocr Disord, doi:10.1186/s12902-020-00626-0
Gebhard, Regitz-Zagrosek, Neuhauser, Morgan, Klein, Impact of sex and gender on COVID-19 outcomes in Europe, Biol Sex Differ, doi:10.1186/s13293-020-00304-9
Goren, Cadegiani, Proxalutamide Reduces the Rate of Hospitalization for COVID-19 Male Outpatients: A Randomized Double-Blind Placebo-Controlled Trial, Front Med, doi:10.3389/fmed.2021.668698
Goren, Va.O-Galv.N S, Wambier, A preliminary observation: Female pattern hair loss among hospitalized COVID-19 patients in Spain -A potential clue to the role of androgens in COVID-19 severity, J Cosmet Dermatol, doi:10.1111/jocd.13443
Goren, Wambier, Herrera, Mccoy, Va.O-Galv.N S et al., Anti-androgens may protect against severe COVID-19 outcomes: results from a prospective cohort study of 77 hospitalized men, J Eur Acad Dermatol Venereol, doi:10.1111/jdv.16953
Guan, Ni, Hu, Clinical Characteristics of Coronavirus Disease 2019 in China, N Engl J Med, doi:10.1056/NEJMoa2002032
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Kyrou, Karteris, Robbins, Polycystic ovary syndrome (PCOS) and COVID-19: an overlooked fefemale patient population at potentially higher risk during the COVID-19 pandemic, BMC Med, doi:10.1186/s12916-020-01697-5
Lucas, Heinlein, Kim, The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis, Cancer Discov, doi:10.1158/2159-8290.CD-13-1010
Mccoy, Cadegiani, Wambier, Herrera, Va.O-Galv.N S et al., 5-Alpha-Reductase Inhibitors are Associated with Reduced Frequency of COVID-19 Symptoms in Females with Androgenetic Alopecia, J Eur Acad Dermatol Venereol, doi:10.1111/jdv.17021
Mccoy, Cadegiani, Wambier, Herrera, Vaño-Galván et al., 5-alpha-reductase inhibitors are associated with reduced frequency of COVID-19 symptoms in males with androgenetic alopecia, J Eur Acad Dermatol Venereol, doi:10.1111/jdv.17021
Mccoy, Wambier, Vano-Galvan, Racial variations in COVID-19 deaths may be due to androgen receptor genetic variants associated with prostate cancer and androgenetic alopecia. Are anti-androgens a potential treatment for COVID-19?, J Cosmet Dermatol, doi:10.1111/jocd.13455
Mccoy, Wambier, Vano-Galvan, Racial variations in COVID-19 deaths may be due to androgen receptor genetic variants associated with prostate cancer and androgenetic alopecia. Are anti-androgens a potential treatment for COVID-19?, J Cosmet Dermatol, doi:10.1111/jocd.13455
Montopoli, Zumerle, Vettor, Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (n=4532)
Montopoli, Zumerle, Vettor, Rugge, Zorzi et al., Androgendeprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a populationbased study (n=4532), Annals of Oncology, doi:10.1016/j.annonc.2020.04.479
Rocco, Silva, Cruz, Early use of nitazoxanide in mild Covid-19 disease: randomised, placebo-controlled trial, Eur Respir J, doi:10.1183/13993003.03725-2020
Sang, Wang, Zhong, Gu, Wang et al., Quantitative determination of proxalutamide in rat plasma and tissues using liquid chromatography/tandem mass spectrometry, Rapid Commun Mass Spectrom, doi:10.1002/rcm.9003
Subramanian, Anand, Adderley, Okoth, Toulis et al., Increased COVID-19 infections in women with polycystic ovary syndrome: a population-based study, Eur J Endocrinol, doi:10.1530/EJE-20-1163
Wambier, Va.O-Galv.N S, Mccoy, Androgenetic Alopecia Present in the Majority of Hospitalized COVID-19 Patients -the "Gabrin sign
Wambier, Vaño-Galván, Mccoy, Pai, Dhurat et al., Androgenetic alopecia in COVID-19: compared to age-matched epidemiologic studies and hospital outcomes with or without the Gabrin sign, J Am Acad Dermatol, doi:10.1016/j.jaad.2020.07.099
DOI record:
{
"DOI": "10.1101/2021.07.06.21260086",
"URL": "http://dx.doi.org/10.1101/2021.07.06.21260086",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>Antiandrogens were shown to be effective in mild-tomoderate COVID-19 male patients, supported by the SARS-CoV-2 dependency on transmembrane protease, serine 2 (TMPRSS2), which is solely modulated by androgens, for cell entry. While women with hyperandrogenism experiment more symptoms in COVID-19 compared to women without hyperandrogenism, and the chronic use of an antiandrogen seemed to mitigate these symptoms, whether the benefits would be observed in overall females is unknown. The objective of this study is to evaluate the efficacy of proxalutamide as a treatment for mild-to-moderate COVID-19 in females.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>This was a randomized, double-blind, placebo-controlled, two-arm, parallel study on COVID-19 female outpatients, that compared the use of proxalutamide versus placebo. The primary outcome was hospitalization rates throughout 30 days after randomization. Patients with laboratory confirmed COVID-19 not hospitalized were recruited in two sites in Brasilia, Brazil, between January 4 and February 28, 2021, were randomized on a 2:3 ratio between proxalutamide and placebo, and were administered proxalutamide 200mg/day or placebo for seven days, in addition to usual care.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>A total of 177 women were randomized, including 75 in the proxalutamide arm and 102 patients in the placebo arm. None of the patients lost follow-up or discontinued treatment. The 30-day hospitalization rate was 2.7% in the proxalutamide arm and 18.6% in the placebo arm (p<0.001), with a hospitalization risk ratio (RR) of 0.14 [95% confidence interval (CI), 0.03-0.59].</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>These findings suggest that treatment of COVID-19 patients with proxalutamide in combination with standard of care was reduced hospitalization rate by 86% (p < 0.001), with no safety concerns. (Clinicaltrials.gov: <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"clintrialgov\" xlink:href=\"NCT04853134\">NCT04853134</jats:ext-link>)</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2021,
7,
10
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0002-2699-4344",
"affiliation": [],
"authenticated-orcid": false,
"family": "Cadegiani",
"given": "Flávio Adsuara",
"sequence": "first"
},
{
"affiliation": [],
"family": "Zimerman",
"given": "Ricardo Ariel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "do Nascimento Fonseca",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "do Nascimento Correia",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McCoy",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wambier",
"given": "Carlos Gustavo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goren",
"given": "Andy",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
7,
10
]
],
"date-time": "2021-07-10T22:10:12Z",
"timestamp": 1625955012000
},
"deposited": {
"date-parts": [
[
2021,
7,
12
]
],
"date-time": "2021-07-12T12:25:16Z",
"timestamp": 1626092716000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2024,
3,
21
]
],
"date-time": "2024-03-21T09:44:25Z",
"timestamp": 1711014265973
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 6,
"issued": {
"date-parts": [
[
2021,
7,
10
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.07.06.21260086",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
7,
10
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
7,
10
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1056/NEJMoa2002032",
"doi-asserted-by": "publisher",
"key": "2021071205250531000_2021.07.06.21260086v1.1"
},
{
"DOI": "10.1186/s13293-020-00304-9",
"doi-asserted-by": "publisher",
"key": "2021071205250531000_2021.07.06.21260086v1.2"
},
{
"DOI": "10.1016/j.jaad.2020.05.079",
"doi-asserted-by": "publisher",
"key": "2021071205250531000_2021.07.06.21260086v1.3"
},
{
"DOI": "10.1111/jocd.13443",
"doi-asserted-by": "publisher",
"key": "2021071205250531000_2021.07.06.21260086v1.4"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"doi-asserted-by": "publisher",
"key": "2021071205250531000_2021.07.06.21260086v1.5"
},
{
"DOI": "10.1158/2159-8290.CD-13-1010",
"doi-asserted-by": "publisher",
"key": "2021071205250531000_2021.07.06.21260086v1.6"
},
{
"DOI": "10.1016/j.jaad.2020.07.099",
"doi-asserted-by": "crossref",
"key": "2021071205250531000_2021.07.06.21260086v1.7",
"unstructured": "Wambier CG , Vaño-Galván S , McCoy J , Pai S , Dhurat R , Goren A , Androgenetic alopecia in COVID-19: compared to age-matched epidemiologic studies and hospital outcomes with or without the Gabrin sign. J Am Acad Dermatol. 2020, doi: https://doi.org/10.1016/j.jaad.2020.07.099."
},
{
"DOI": "10.1111/jocd.13455",
"doi-asserted-by": "publisher",
"key": "2021071205250531000_2021.07.06.21260086v1.8"
},
{
"DOI": "10.1111/jocd.13455",
"doi-asserted-by": "publisher",
"key": "2021071205250531000_2021.07.06.21260086v1.9"
},
{
"DOI": "10.1111/jdv.16953",
"doi-asserted-by": "publisher",
"key": "2021071205250531000_2021.07.06.21260086v1.10"
},
{
"DOI": "10.1016/j.annonc.2020.04.479",
"doi-asserted-by": "publisher",
"key": "2021071205250531000_2021.07.06.21260086v1.11"
},
{
"DOI": "10.1016/j.annonc.2020.04.479",
"doi-asserted-by": "crossref",
"key": "2021071205250531000_2021.07.06.21260086v1.12",
"unstructured": "Montopoli M , Zumerle S , Vettor R , Rugge M , Zorzi M , Catapano CV , Carbone GM , Cavalli A , Pagano F , Ragazzi E , Prayer-Galetti T , Alimonti A , Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a populationbased study (n=4532), Annals of Oncology (2020), doi: https://doi.org/10.1016/j.annonc.2020.04.479."
},
{
"DOI": "10.1111/jdv.17021",
"doi-asserted-by": "publisher",
"key": "2021071205250531000_2021.07.06.21260086v1.13"
},
{
"DOI": "10.7759/cureus.13047",
"doi-asserted-by": "publisher",
"key": "2021071205250531000_2021.07.06.21260086v1.14"
},
{
"DOI": "10.1186/s12916-020-01697-5",
"doi-asserted-by": "crossref",
"key": "2021071205250531000_2021.07.06.21260086v1.15",
"unstructured": "Kyrou I , Karteris E , Robbins T , et al. Polycystic ovary syndrome (PCOS) and COVID-19: an overlooked fefemale patient population at potentially higher risk during the COVID-19 pandemic. BMC Med 2020;18(220). https://doi.org/10.1186/s12916-020-01697-5"
},
{
"DOI": "10.1111/jdv.17004",
"doi-asserted-by": "publisher",
"key": "2021071205250531000_2021.07.06.21260086v1.16"
},
{
"DOI": "10.1530/EJE-20-1163",
"doi-asserted-by": "publisher",
"key": "2021071205250531000_2021.07.06.21260086v1.17"
},
{
"DOI": "10.1111/jdv.17021",
"doi-asserted-by": "publisher",
"key": "2021071205250531000_2021.07.06.21260086v1.18"
},
{
"DOI": "10.1186/s12902-020-00626-0",
"doi-asserted-by": "publisher",
"key": "2021071205250531000_2021.07.06.21260086v1.19"
},
{
"DOI": "10.1002/rcm.9003",
"doi-asserted-by": "publisher",
"key": "2021071205250531000_2021.07.06.21260086v1.20"
},
{
"DOI": "10.3389/fmed.2021.668698",
"doi-asserted-by": "publisher",
"key": "2021071205250531000_2021.07.06.21260086v1.21"
},
{
"key": "2021071205250531000_2021.07.06.21260086v1.22",
"unstructured": "Rocco PRM , Silva PL , Cruz FF , et al. Early use of nitazoxanide in mild Covid-19 disease: randomised, placebo-controlled trial. Eur Respir J 2020; in press (https://doi.org/10.1183/13993003.03725-2020)"
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2021.07.06.21260086"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Proxalutamide (GT0918) Reduces the Rate of Hospitalization in mild-to-moderate COVID-19 Female Patients: A Randomized Double-Blinded Placebo-Controlled Two-Arm Parallel Trial",
"type": "posted-content"
}
cadegiani5