Proxalutamide (GT0918) Reduces the Rate of Hospitalization for COVID-19 Male Outpatients: A Randomized Double-Blinded Placebo-Controlled Trial
et al., Frontiers in Medicine, doi:10.3389/fmed.2021.668698, NCT04446429, Dec 2020 (preprint)
RCT 268 male patients in Brazil, 134 treated with proxalutamide, showing significantly lower hospitalization and mechanical ventilation.
This paper was retracted, however no specific reason is provided, the editors have ignored the authors, and the "external expert" was reportedly funded by Pfizer. For details see1.
The retraction notice states: "The investigation found that the claims made in the conclusions were not adequately supported by the methodology of the study. In particular, as confirmed by an external expert, the process of allocation to treatment and control was not sufficiently random."
The lack of any detail on what conclusion is not supported and why, or details of any issues in randomization, suggests the paper was censored rather than retracted.
|
risk of death, 80.0% lower, RR 0.20, p = 0.50, treatment 0 of 134 (0.0%), control 2 of 134 (1.5%), NNT 67, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of mechanical ventilation, 97.1% lower, RR 0.03, p < 0.001, treatment 0 of 134 (0.0%), control 17 of 134 (12.7%), NNT 7.9, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of hospitalization, 91.0% lower, RR 0.09, p < 0.001, treatment 3 of 134 (2.2%), control 35 of 134 (26.1%), NNT 4.2.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
McCoy et al., 30 Dec 2020, Double Blind Randomized Controlled Trial, Brazil, peer-reviewed, 15 authors, study period 15 June, 2020 - 28 July, 2020, censored, see details, trial NCT04446429 (history).
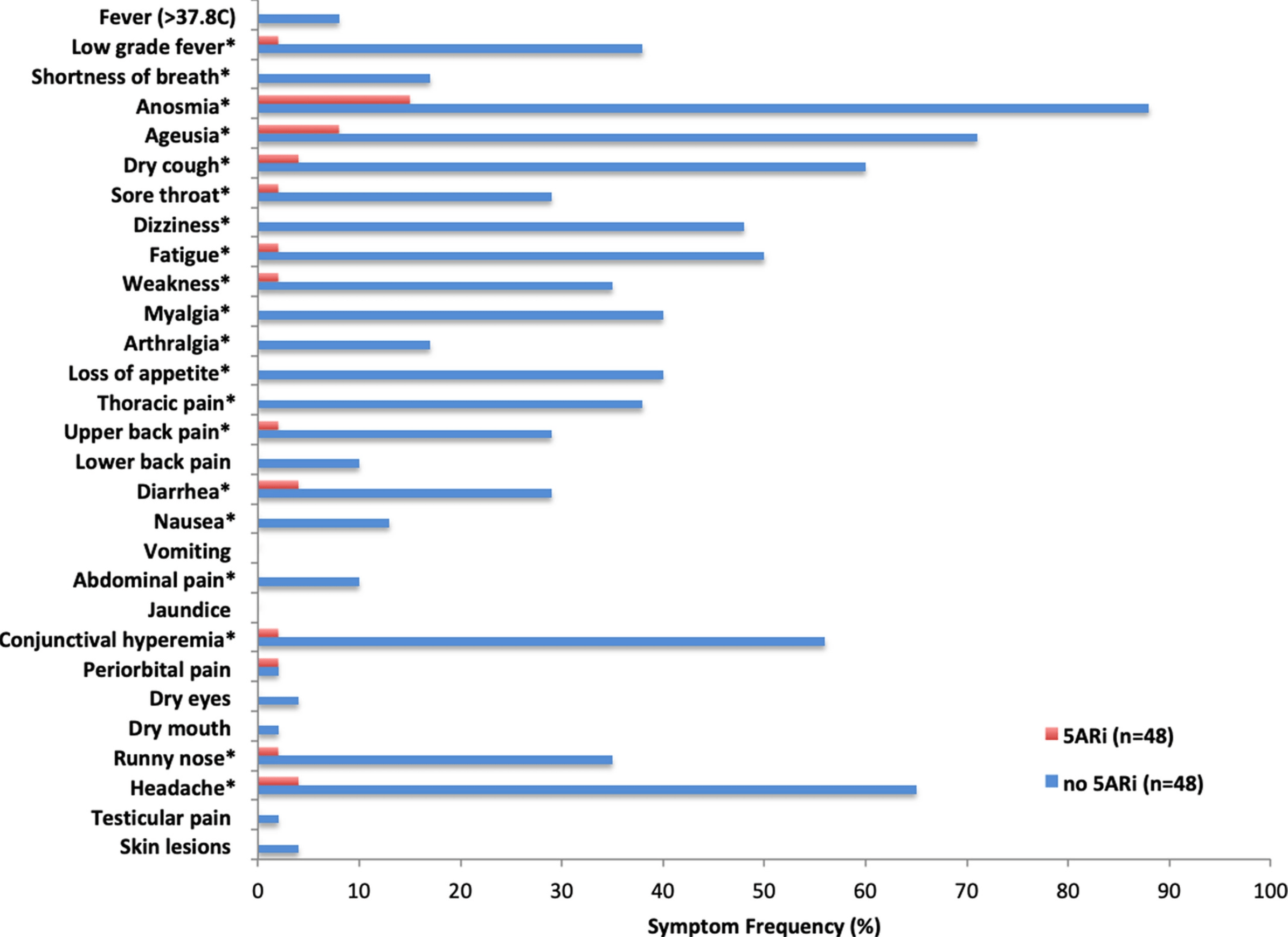
5-alpha-reductase inhibitors are associated with reduced frequency of COVID-19 symptoms in males with androgenetic alopecia
We have previously reported that men with androgenetic alopecia (AGA) are more likely to present with severe COVID-19 symptoms, potentially implicating androgen sensitivity as a risk factor for COVID-19. [1] [2] [3] As such, we hypothesized that 5-alphareductase inhibitors (5ARi) may reduce the severity of COVID-19 disease. To test this hypothesis, we conducted a retrospective cohort analysis on male subjects with laboratory confirmed SARS-CoV-2 infection. The subjects presented at one of five outpatient clinics (Corpometria Institute Brasilia, Brazil) from 15 June to 28 July 2020. At the time of visit, 29 clinical symptoms associated with SARS-CoV-2 infection were documented. For analysis, male subjects with AGA were selected. The frequency of clinical symptoms in males with AGA using 5ARis was compared to those not using 5ARis. Among the men presenting at the clinic, 300 were positive for SARS-CoV-2. Of these, 65 had AGA but were not using 5ARis, and 48 had AGA and were taking a 5ARi for at least six months prior to the study. The only 5ARi used in this cohort was dutasteride (0.5 mg) for the treatment of AGA. Propensity score
Conflicts of interest
References
Clark, Hermann, Cunningham, Wilson, Morrill et al., Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5a-reductase inhibitor, J Clin Endocrinol Metab, doi:10.1111/jdv.17021
Gebhard, Regitz-Zagrosek, Neuhauser, Morgan, Klein, Impact of sex and gender on COVID-19 outcomes in Europe, Biol Sex Differ
Goren, Vaño-Galv An, Wambier, A preliminary observation: male pattern hair loss among hospitalized COVID-19 patients in Spain -a potential clue to the role of androgens in COVID-19 severity, J Cosmet Dermatol
Montopoli, Zumerle, Vettor, Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a populationbased study (n=4532), Ann Oncol, doi:10.1016/j.annonc.2020.04.479
Wambier, Vaño-Galv An, Mccoy, Androgenetic alopecia present in the majority of hospitalized COVID-19 patients -the "Gabrin sign, J Am Acad Dermatol, doi:10.1016/j.jaad.2020.05.079
Wambier, Vaño-Galv An, Mccoy, Pai, Dhurat et al., Androgenetic alopecia in COVID-19: compared to age-matched epidemiologic studies and hospital outcomes with or without the Gabrin sign, J Am Acad Dermatol, doi:10.1016/j.jaad.2020.07.099
DOI record:
{
"DOI": "10.3389/fmed.2021.668698",
"ISSN": [
"2296-858X"
],
"URL": "http://dx.doi.org/10.3389/fmed.2021.668698",
"abstract": "<jats:p>Antiandrogens have demonstrated a protective effect for COVOD-19 patients in observational and interventional studies. The goal of this study was to determine if proxalutamide, an androgen receptor antagonist, could be an effective treatment for men with COVID-19 in an outpatient setting. A randomized, double-blinded, placebo-controlled clinical trial was conducted at two outpatient centers (Brasilia, Brazil). Patients were recruited from October 21 to December 24, 2020 (<jats:ext-link>clinicaltrials.gov</jats:ext-link> number, NCT04446429). Male patients with confirmed COVID-19 but not requiring hospitalization (COVID-19 8-point ordinal scale &lt;3) were administered proxalutamide 200 mg/day or placebo for up to 7 days. The primary endpoint was hospitalization rate at 30 days post-randomization. A total of 268 men were randomized in a 1:1 ratio. 134 patients receiving proxalutamide and 134 receiving placebo were included in the intention-to-treat analysis. The 30-day hospitalization rate was 2.2% in men taking proxalutamide compared to 26% in placebo, <jats:italic>P</jats:italic> &lt; 0.001. The 30-day hospitalization risk ratio was 0.09; 95% confidence interval (CI) 0.03–0.27. Patients in the proxalutamide arm more frequently reported gastrointestinal adverse events, however, no patient discontinued treatment. In placebo group, 6 patients were lost during follow-up, and 2 patients died from acute respiratory distress syndrome. Here we demonstrate the hospitalization rate in proxalutamide treated men was reduced by 91% compared to usual care.</jats:p>",
"alternative-id": [
"10.3389/fmed.2021.668698"
],
"author": [
{
"affiliation": [],
"family": "McCoy",
"given": "John",
"sequence": "first"
},
{
"affiliation": [],
"family": "Goren",
"given": "Andy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cadegiani",
"given": "Flávio Adsuara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vaño-Galván",
"given": "Sergio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kovacevic",
"given": "Maja",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Situm",
"given": "Mirna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shapiro",
"given": "Jerry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sinclair",
"given": "Rodney",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tosti",
"given": "Antonella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stanimirovic",
"given": "Andrija",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fonseca",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dorner",
"given": "Edinete",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Onety",
"given": "Dirce Costa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zimerman",
"given": "Ricardo Ariel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wambier",
"given": "Carlos Gustavo",
"sequence": "additional"
}
],
"container-title": "Frontiers in Medicine",
"container-title-short": "Front. Med.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2021,
7,
19
]
],
"date-time": "2021-07-19T06:47:44Z",
"timestamp": 1626677264000
},
"deposited": {
"date-parts": [
[
2024,
4,
3
]
],
"date-time": "2024-04-03T13:23:08Z",
"timestamp": 1712150588000
},
"indexed": {
"date-parts": [
[
2024,
4,
3
]
],
"date-time": "2024-04-03T13:43:31Z",
"timestamp": 1712151811076
},
"is-referenced-by-count": 33,
"issued": {
"date-parts": [
[
2021,
7,
19
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
19
]
],
"date-time": "2021-07-19T00:00:00Z",
"timestamp": 1626652800000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2021.668698/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2021,
7,
19
]
]
},
"published-online": {
"date-parts": [
[
2021,
7,
19
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1073/pnas.2021450118",
"article-title": "Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and TMPRSS2",
"author": "Qiao",
"doi-asserted-by": "publisher",
"first-page": "e2021450118",
"journal-title": "Proc Natl Acad Sci USA.",
"key": "B1",
"volume": "118",
"year": "2020"
},
{
"DOI": "10.1371/journal.ppat.1009212",
"article-title": "Hydroxychloroquine-mediated inhibition of SARS-CoV-2 entry is attenuated by TMPRSS2",
"author": "Ou",
"doi-asserted-by": "publisher",
"first-page": "e1009212",
"journal-title": "PLOS Pathog.",
"key": "B2",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.1016/j.stem.2020.11.009",
"article-title": "Androgen signaling regulates SARS-CoV-2 receptor levels and is associated with severe COVID-19 symptoms in men",
"author": "Samuel",
"doi-asserted-by": "publisher",
"first-page": "876",
"journal-title": "Cell Stem Cell.",
"key": "B3",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1016/j.jaad.2020.04.032",
"article-title": "Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is likely to be androgen mediated",
"author": "Wambier",
"doi-asserted-by": "publisher",
"first-page": "308",
"journal-title": "J Am Acad Dermatol.",
"key": "B4",
"volume": "83",
"year": "2020"
},
{
"DOI": "10.1016/j.jaad.2020.07.099",
"article-title": "Androgenetic alopecia in COVID-19: compared to age-matched epidemiologic studies and hospital outcomes with or without the Gabrin sign",
"author": "Wambier",
"doi-asserted-by": "publisher",
"first-page": "e453",
"journal-title": "J Am Acad Dermatol.",
"key": "B5",
"volume": "83",
"year": "2020"
},
{
"DOI": "10.1016/j.jaad.2020.07.062",
"article-title": "Male balding is a major risk factor for severe COVID-19",
"author": "Lee",
"doi-asserted-by": "publisher",
"first-page": "1359",
"journal-title": "J Am Acad Dermatol.",
"key": "B6",
"volume": "68",
"year": "2020"
},
{
"DOI": "10.1111/exd.14220",
"article-title": "Alopecia and gray hair are associated with COVID-19 severity",
"author": "Ramos",
"doi-asserted-by": "publisher",
"first-page": "1250",
"journal-title": "Exp Dermatol.",
"key": "B7",
"volume": "29",
"year": "2020"
},
{
"article-title": "Alopecia and severity of COVID-19: a cross-sectional study in Peru",
"author": "Salazar Arenas",
"first-page": "37",
"journal-title": "Le Infez Med.",
"key": "B8",
"volume": "29",
"year": "2021"
},
{
"DOI": "10.1016/j.annonc.2020.04.479",
"article-title": "Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532)",
"author": "Montopoli",
"doi-asserted-by": "publisher",
"first-page": "1040",
"journal-title": "Ann Oncol.",
"key": "B9",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1111/jdv.16953",
"article-title": "Anti-androgens may protect against severe COVID-19 outcomes: results from a prospective cohort study of 77 hospitalized men",
"author": "Goren",
"doi-asserted-by": "publisher",
"first-page": "e13",
"journal-title": "J Eur Acad Dermatol Venereol.",
"key": "B10",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1111/jdv.17021",
"article-title": "5-alpha-reductase inhibitors are associated with reduced frequency of COVID-19 symptoms in males with androgenetic alopecia",
"author": "McCoy",
"doi-asserted-by": "publisher",
"first-page": "e243",
"journal-title": "J Eur Acad Dermatol Venereol",
"key": "B11",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.7759/cureus.13047",
"article-title": "Early antiandrogen therapy with dutasteride reduces viral shedding, inflammatory responses, and time-to-remission in males with COVID-19: a randomized, double-blind, placebo-controlled interventional trial (EAT-DUTA AndroCoV Trial - Biochemical)",
"author": "Cadegiani",
"doi-asserted-by": "publisher",
"first-page": "e13047",
"journal-title": "Cureus.",
"key": "B12",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.47176/mjiri.35.30",
"article-title": "Finasteride in hospitalized adult males with COVID-19: A risk factor for severity of the disease or an adjunct treatment: A randomized controlled clinical trial TT",
"author": "Zarehoseinzade",
"doi-asserted-by": "publisher",
"first-page": "232",
"journal-title": "MJIRI.",
"key": "B13",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1007/s10637-020-00901-w",
"article-title": "Metabolomic profiling to evaluate the efficacy of proxalutamide, a novel androgen receptor antagonist, in prostate cancer cells",
"author": "Qu",
"doi-asserted-by": "publisher",
"first-page": "1292",
"journal-title": "Invest New Drugs.",
"key": "B14",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.1126/science.1168175",
"article-title": "Development of a second-generation antiandrogen for treatment of advanced prostate cancer",
"author": "Tran",
"doi-asserted-by": "publisher",
"first-page": "787",
"journal-title": "Science.",
"key": "B15",
"volume": "324",
"year": "2009"
},
{
"DOI": "10.7759/cureus.13492",
"article-title": "Proxalutamide significantly accelerates viral clearance and reduces time to clinical remission in patients with mild to moderate COVID-19: results from a randomized, double-blinded, placebo-controlled trial",
"author": "Cadegiani",
"doi-asserted-by": "publisher",
"first-page": "e13492",
"journal-title": "Cureus",
"key": "B16",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.2807/1560-7917.ES.2020.25.49.2000790",
"article-title": "Epidemiological characteristics of COVID-19 cases in Italy and estimates of the reproductive numbers one month into the epidemic",
"author": "Riccardo",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv.",
"key": "B17",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1590/S0365-05962012000400012",
"article-title": "Brazilian blood donation eligibility criteria for dermatologic patients",
"author": "Wambier",
"doi-asserted-by": "publisher",
"first-page": "590",
"journal-title": "An Bras Dermatol.",
"key": "B18",
"volume": "87",
"year": "2012"
},
{
"DOI": "10.1016/j.jaad.2021.03.084",
"article-title": "Response to “Reply to effectiveness of dutasteride in a large series of patients with FFA in real clinical practice",
"author": "Pindado-Ortega",
"doi-asserted-by": "publisher",
"journal-title": "J Am Acad Dermatol",
"key": "B19",
"year": "2021"
},
{
"DOI": "10.1016/j.ejca.2020.04.013",
"article-title": "Preclinical profile and phase I clinical trial of a novel androgen receptor antagonist GT0918 in castration-resistant prostate cancer",
"author": "Zhou",
"doi-asserted-by": "publisher",
"first-page": "29",
"journal-title": "Eur J Cancer.",
"key": "B20",
"volume": "134",
"year": "2020"
},
{
"DOI": "10.1136/bcr-2021-241572",
"article-title": "Potential risk for developing severe COVID-19 disease among anabolic steroid users",
"author": "Cadegiani",
"doi-asserted-by": "publisher",
"first-page": "241572",
"journal-title": "BMJ Case Rep.",
"key": "B21",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "271",
"journal-title": "Cell.",
"key": "B22",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1101/2020.09.13.295691",
"article-title": "Ambroxol hydrochloride inhibits the interaction between severe acute respiratory syndrome coronavirus 2 spike protein's receptor binding domain and recombinant human ACE2",
"author": "Olaleye",
"doi-asserted-by": "publisher",
"journal-title": "bioRxiv Prepr Serv Biol [preprint]",
"key": "B23",
"year": "2020"
},
{
"DOI": "10.1038/s41467-021-21171-x",
"article-title": "Distinct mechanisms for TMPRSS2 expression explain organ-specific inhibition of SARS-CoV-2 infection by enzalutamide",
"author": "Li",
"doi-asserted-by": "publisher",
"first-page": "866",
"journal-title": "Nat Commun.",
"key": "B24",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1242/jcs.02786",
"article-title": "Androgens modulate the inflammatory response during acute wound healing",
"author": "Gilliver",
"doi-asserted-by": "publisher",
"first-page": "722",
"journal-title": "J Cell Sci.",
"key": "B25",
"volume": "119",
"year": "2006"
},
{
"DOI": "10.1152/japplphysiol.00012.2008",
"article-title": "Flutamide protects against trauma-hemorrhage-induced liver injury via attenuation of the inflammatory response, oxidative stress, and apopotosis",
"author": "Kan",
"doi-asserted-by": "publisher",
"first-page": "595",
"journal-title": "J Appl Physiol.",
"key": "B26",
"volume": "105",
"year": "2008"
},
{
"DOI": "10.1186/1471-2407-9-92",
"article-title": "Androgen deprivation modulates the inflammatory response induced by irradiation",
"author": "Wu",
"doi-asserted-by": "publisher",
"first-page": "92",
"journal-title": "BMC Cancer.",
"key": "B27",
"volume": "9",
"year": "2009"
},
{
"DOI": "10.1007/978-3-030-55617-4_1",
"article-title": "Interleukin-6 function and targeting in prostate cancer",
"author": "Culig",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Adv Exp Med Biol.",
"key": "B28",
"volume": "1290",
"year": "2021"
},
{
"DOI": "10.1101/2021.04.19.21255441",
"article-title": "Early treatment with nitazoxanide prevents worsening of mild and moderate COVID-19 and subsequent hospitalization",
"author": "Rossignol",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv [preprint].",
"key": "B29",
"year": "2021"
},
{
"DOI": "10.1183/13993003.03725-2020",
"article-title": "Early use of nitazoxanide in mild Covid-19 disease: randomised, placebo-controlled trial",
"author": "Rocco",
"doi-asserted-by": "publisher",
"first-page": "2003725",
"journal-title": "Eur Respir J.",
"key": "B30",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1002/jmv.26880",
"article-title": "Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-19",
"author": "Elalfy",
"doi-asserted-by": "publisher",
"first-page": "3176",
"journal-title": "J Med Virol.",
"key": "B31",
"volume": "93",
"year": "2021"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2021.668698/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "RETRACTED: Proxalutamide Reduces the Rate of Hospitalization for COVID-19 Male Outpatients: A Randomized Double-Blinded Placebo-Controlled Trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "8"
}
mccoy