Final Results of a Randomized, Placebo-Controlled, Two-Arm, Parallel Clinical Trial of Proxalutamide for Hospitalized COVID-19 Patients: A Multiregional, Joint Analysis of the Proxa-Rescue AndroCoV Trial
et al., Cureus, doi:10.7759/cureus.20691, NCT04728802, Dec 2021
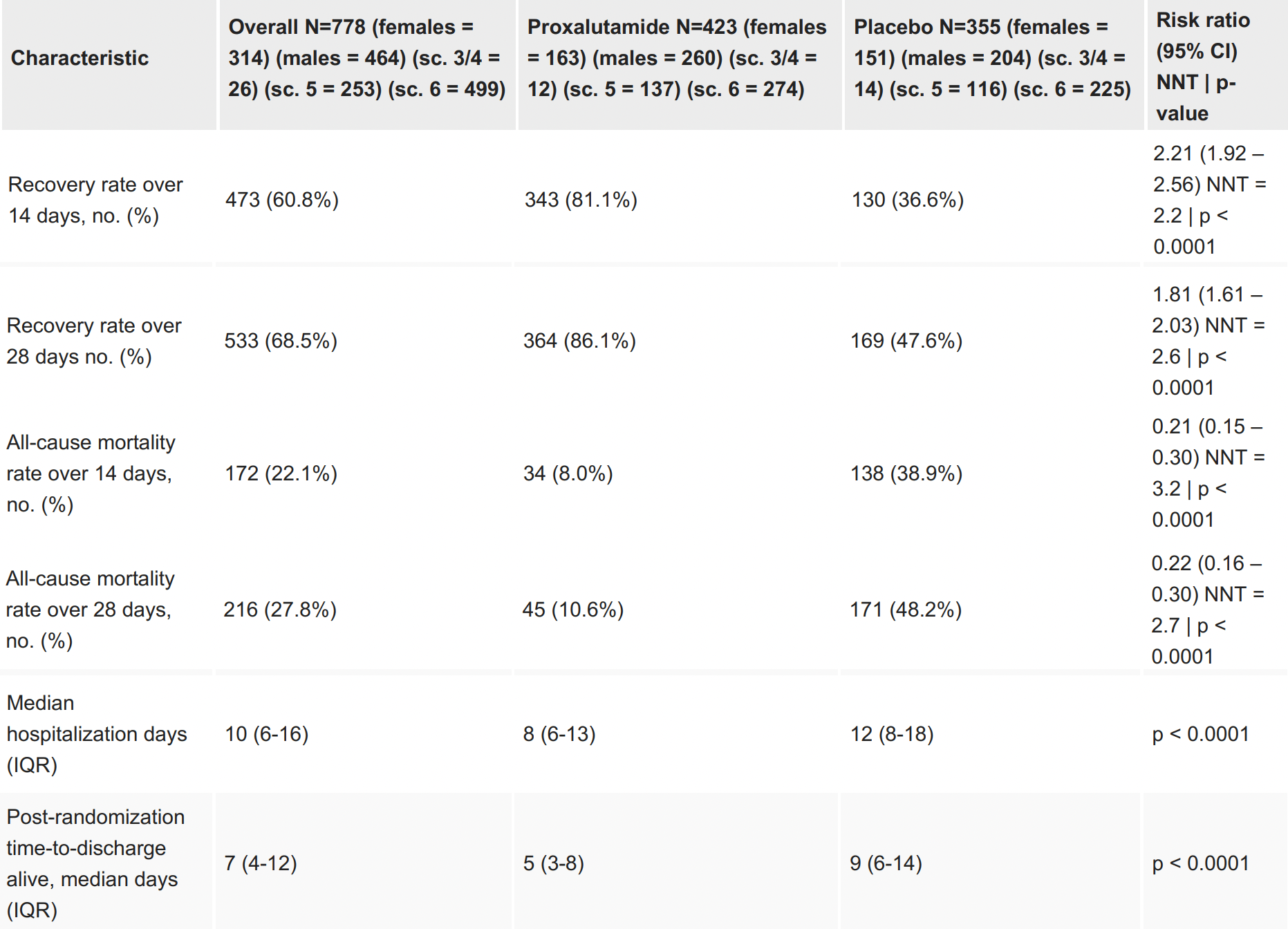
RCT 778 hospitalized patients in Brazil, 423 treated with proxalutamide, showing significantly lower mortality and improved recovery with treatment. NCT04728802 (history) and NCT05126628 (history). Authors note that cases in this trial were predominantly the P.1 Gamma variant, for which proxalutamide may be more effective compared to other variants.
|
risk of death, 78.0% lower, RR 0.22, p < 0.001, treatment 45 of 423 (10.6%), control 171 of 355 (48.2%), NNT 2.7, adjusted per study, 28 days, Cox proportional hazards.
|
|
risk of death, 79.0% lower, RR 0.21, p < 0.001, treatment 34 of 423 (8.0%), control 138 of 355 (38.9%), NNT 3.2, adjusted per study, 14 days, Cox proportional hazards.
|
|
recovery rate, RR 0.55, p < 0.001, treatment 423, control 355, adjusted per study, inverted to make RR<1 favor treatment, 28 days, Cox proportional hazards.
|
|
recovery rate, RR 0.45, p < 0.001, treatment 423, control 355, adjusted per study, inverted to make RR<1 favor treatment, 14 days, Cox proportional hazards, primary outcome.
|
|
hospitalization time, 33.3% lower, relative time 0.67, p < 0.001, treatment 423, control 355.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Cadegiani et al., 25 Dec 2021, Double Blind Randomized Controlled Trial, Brazil, peer-reviewed, 15 authors, study period 1 February, 2021 - 15 April, 2021, trial NCT04728802 (history).
Final Results of a Randomized, Placebo-Controlled, Two-Arm, Parallel Clinical Trial of Proxalutamide for Hospitalized COVID-19 Patients: A Multiregional, Joint Analysis of the Proxa-Rescue AndroCoV Trial
Cureus, doi:10.7759/cureus.20691
Background The role of androgens on COVID-19 is well established. Proxalutamide is a second-generation, nonsteroidal antiandrogen (NSAA) with the highest antiandrogen potency among NSAAs and concurrent regulation of angiotensin-converting enzyme 2 (ACE2) expression and inflammatory response. Proxalutamide has been demonstrated to be effective to prevent hospitalizations in early COVID-19 in randomized clinical trials (RCTs). Conversely, in hospitalized COVID-19 patients, preliminary results from two different arms of an RCT (The Proxa-Rescue AndroCoV Trial) also demonstrated a reduction in all-cause mortality. This study aims to report the final, joint results of the two arms (North arm and South arm) of the Proxa-Rescue AndroCoV trial of the two arms (North and South arms) combined, and to evaluate whether COVID-19 response to proxalutamide was consistent across different regions (Northern Brazil and Southern Brazil).
Materials and methods Upon randomization, hospitalized COVID-19 patients received either proxalutamide 300mg/day or placebo for 14 days, in addition to usual care, in a proxalutamide:placebo ratio of 1:1 in the North arm and 4:1 in the South arm (ratio was modified due to preliminary report of high drug efficacy). Datasets of the South and North arms were combined, and statistical analysis was performed for the overall study population. Proxalutamide was compared to placebo group for 14-day and 28-day recovery (discharge alive from the hospital) and mortality rates, and overall and post-randomization hospitalization stay. Results of proxalutamide and placebo groups were also compared between the North and South arms. Analysis was also performed stratified by sex and baseline WHO COVID Ordinary Score.
Results A total of 778 subjects were included (645 from the North, 317 from the proxalutamide group and 328 from the placebo group; 133 from the South arm, 106 from the proxalutamide group and 27 from the placebo group). Recovery rate was 121% higher in proxalutamide than placebo group at day 14 [81.1% vs 36.6%; Recovery ratio (RecR) 2.21; 95% confidence interval (95% CI), 1.92-2.56; p<0.0001], and 81% higher at day 28 (98.1% vs 47.6%; RecR, 1.81; 95% CI, 1.61-2.03; p<0.0001). All-cause mortality rate was 80% lower in proxalutamide than placebo group at Day 14 [8.0% vs 39.2%; Risk ratio (RR), 0.20; 95% CI, 0.14-0.29; p<0.0001], and 78% lower at Day 28 (10.6% vs 48.2%; RR, 0.22; 95% CI 0.16-0.30). Post-randomization timeto-discharge was shorter in proxalutamide [median, 5 days; interquartile range (IQR), 3-8] than placebo group (median, 9 days; IQR, 6-14) (p<0.0001). Results were statistically similar between North and South arms for all measured outcomes. Males and females presented similar results in all outcomes. Patients that did not require oxygen use (scores 3 and 4) did not present statistically significant improvement in recovery and mortality rates, whereas scores 5 and 6 presented significant improvements in..
Additional Information Disclosures Human subjects: Consent was obtained or waived by all participants in this study. National Ethics Committee (CEP/CONEP/MS) issued approval 4.513.425. The present study was approved by the National Ethics Committee (CEP/CONEP/MS), unrestricted to a specific site, once the research protocol was followed as approved and the study conducted until September 3, 2021, when the approval number to continue the study, in case the study was ongoing, was ceased (the study ended in April 16, 2021) . Approval number 4.513.425; process number (CAAE) 41909121.0.0000.5553. This trial is registered in clinicaltrials.gov under two different numbers, one for each arm. (North arm, NCT04728802; South arm, NCT05126628). Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: Kintor Pharmaceuticals, Ltd., the manufacturer of proxalutamide, provided the drugs for this trial, and is conducting phase 3 trials for COVID-19. In case efficacy of..
References
Afar, Vivanco, Hubert, Catalytic cleavage of the androgen-regulated TMPRSS2 protease results in its secretion by prostate and prostate cancer epithelia, Cancer Res
Bhowmick, Oft, Dorff, COVID-19 and androgen-targeted therapy for prostate cancer patients, Endocr Relat Cancer, doi:10.1530/ERC-20-0165
Blum, Cimerman, Hunter, Nitazoxanide superiority to placebo to treat moderate COVID-19 -A Pilot prove of concept randomized double-blind clinical trial, EClinicalMedicine, doi:10.1016/j.eclinm.2021.100981
Breithaupt-Faloppa, De, Correia, Prado, Stilhano et al., Estradiol, a potential ally to alleviate SARS-CoV-2 infection, Clinics, doi:10.6061/clinics/2020/e1980
Cadegiani, Fonseca, Correia, Proxalutamide improves lung injury in hospitalized COVID-19 patients -an analysis of the radiological findings of the Proxa-Rescue AndroCoV trial, PREPRINT, doi:10.1101/2021.07.01.21259656
Cadegiani, Fonseca, Mccoy, Efficacy of proxalutamide in hospitalized COVID-19 patients: a randomized, double-blind, placebo-controlled, parallel-design clinical trial, PREPRINT, doi:10.1101/2021.06.22.21259318
Cadegiani, Goren, Wambier, Mccoy, Early COVID-19 therapy with azithromycin plus nitazoxanide, ivermectin or hydroxychloroquine in outpatient settings significantly improved COVID-19 outcomes compared to known outcomes in untreated patients, New Microbes New Infect, doi:10.1016/j.nmni.2021.100915
Cadegiani, Goren, Wambier, Zimerman, Proxalutamide improves inflammatory, immunologic, and thrombogenic markers in mild-to-moderate COVID-19 males and females: an exploratory analysis of a randomized, double-blinded, placebo-controlled trial early antiandrogen therapy (EAT) with proxalutamide (The EAT-Proxa Biochemical AndroCoV-Trial), PREPRINT), doi:10.1101/2021.07.24.21261047
Cadegiani, Lim, Goren, Clinical symptoms of hyperandrogenic women diagnosed with COVID-19, J Eur Acad Dermatol Venereol, doi:10.1111/jdv.17004
Cadegiani, Lin, Goren, Wambier, Potential risk for developing severe COVID-19 disease among anabolic steroid users, BMJ Case Rep, doi:10.1136/bcr-2021-241572
Cadegiani, Mccoy, Wambier, Goren, Early antiandrogen therapy with dutasteride reduces viral shedding, inflammatory responses, and time-to-remission in males with COVID-19: a randomized, double-blind, placebo-controlled interventional trial (EAT-DUTA AndroCoV Trial -Biochemical), Cureus, doi:10.7759/cureus.13047
Cadegiani, Mccoy, Wambier, Proxalutamide significantly accelerates viral clearance and reduces time to clinical remission in patients with mild to moderate COVID-19: results from a randomized, double-blinded, placebo-controlled trial, Cureus, doi:10.7759/cureus.13492
Cadegiani, Repurposing existing drugs for COVID-19: an endocrinology perspective, BMC Endocr Disord, doi:10.1186/s12902-020-00626-0
Cadegiani, Wambier, Goren, Spironolactone: an anti-androgenic and anti-hypertensive drug that may provide protection against the novel coronavirus (SARS-CoV-2) induced acute respiratory distress syndrome (ARDS) in COVID-19, Front Med, doi:10.3389/fmed.2020.00453
Cadegiani, Zimerman, Fonseca, Correia, Mccoy et al., Proxalutamide (GT0918) reduces the rate of hospitalization in mild-to-moderate COVID-19 female patients: a randomized double-blinded placebo-controlled two-arm parallel trial, PREPRINT, doi:10.1101/2021.07.06.21260086
Dambha-Miller, Hinton, Joy, Feher, De Lusignan, Mortality in COVID-19 amongst women on hormone replacement therapy or combined oral contraception: a cohort study, PREPRINT, doi:10.1101/2021.02.16.21251853
Dhindsa, Zhang, Mcphaul, Association of circulating sex hormones with inflammation and disease severity in patients with COVID-19, JAMA Netw Open, doi:10.1001/jamanetworkopen.2021.11398
Durcan, Turan, Bircan, TransCOVID: does gender-affirming hormone therapy play a role in contracting COVID-19?, J Sex Marital Ther, doi:10.1080/0092623X.2021.2000535
Fanning, Zoulim, Hou, Bertoletti, Therapeutic strategies for hepatitis B virus infection: towards a cure, Nat Rev Drug Discov, doi:10.1038/s41573-019-0037-0
Flávio, Zimerman, Goren, Wambier, Proxalutamide Treatment for Hospitalized COVID-19 Patients in Southern Brazil: The South Arm of a Randomized, Double-Blind, Placebo-Controlled, Parallel Clinical Trial -The South Proxa-Rescue AndroCoV Trial
Franceschi, Caldana, Perin, Predominance of the SARS-CoV-2 lineage P.1 and its sublineage P.1.2 in patients from the metropolitan region of Porto Alegre, Southern Brazil in, Pathogens, doi:10.3390/pathogens10080988
Freitas, Lemos, Beckedorff, Cavalcanti, Siqueira et al., The increase in the risk of severity and fatality rate of covid-19 in southern Brazil after the emergence of the Variant of Concern (VOC) SARS-CoV-2 P.1 was greater among young adults without pre-existing risk conditions, doi:10.1101/2021.04.13.21255281
Gebhard, Regitz-Zagrosek, Neuhauser, Morgan, Klein, Impact of sex and gender on COVID-19 outcomes in Europe, Biol Sex Differ, doi:10.1186/s13293-020-00304-9
Ghandehari, Matusov, Pepkowitz, Progesterone in addition to standard of care vs standard of care alone in the treatment of men hospitalized with moderate to severe COVID-19: a randomized, controlled pilot trial, Chest, doi:10.1016/j.chest.2021.02.024
Goren, Wambier, Herrera, Anti-androgens may protect against severe COVID-19 outcomes: results from a prospective cohort study of 77 hospitalized men, J Eur Acad Dermatol Venereol, doi:10.1111/jdv.16953
Hird, Magee, Bhindi, A systematic review and network meta-analysis of novel androgen receptor inhibitors in non-metastatic castration-resistant prostate cancer, Clin Genitourin Cancer, doi:10.1016/j.clgc.2020.02.005
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Houghton, Hepatitis C virus: 30 years after its discovery, Cold Spring Harb Perspect Med, doi:10.1101/cshperspect.a037069
Kumar, Zuo, Yalavarthi, SARS-CoV-2 spike protein S1-mediated endothelial injury and proinflammatory state is amplified by dihydrotestosterone and prevented by mineralocorticoid antagonism, Viruses, doi:10.3390/v13112209
Leach, Mohr, Giotis, The antiandrogen enzalutamide downregulates TMPRSS2 and reduces cellular entry of SARS-CoV-2 in human lung cells, Nat Commun, doi:10.1038/s41467-021-24342-y
Lee, Heberer, Gao, A population-level analysis of the protective effects of androgen deprivation therapy against COVID-19 disease incidence and severity, PREPRINT, doi:10.1101/2021.05.10.21255146
Lee, Yousaf, Fang, Kolodney, Male balding is a major risk factor for severe COVID-19, J Am Acad Dermatol, doi:10.1016/j.jaad.2020.07.062
Mccoy, Cadegiani, Wambier, 5-alpha-reductase inhibitors are associated with reduced frequency of COVID-19 symptoms in males with androgenetic alopecia, J Eur Acad Dermatol Venereol, doi:10.1111/jdv.17021
Mccoy, Goren, Cadegiani, Proxalutamide reduces the rate of hospitalization for COVID-19 male outpatients: a randomized double-blinded placebo-controlled trial, Front Med, doi:10.3389/fmed.2021.668698
Mccoy, Wambier, Herrera, Androgen receptor genetic variant predicts COVID-19 disease severity: a prospective longitudinal study of hospitalized COVID-19 male patients, J Eur Acad Dermatol Venereol, doi:10.1111/jdv.16956
Mcintosh, COVID-19 clinical features
Montopoli, Zumerle, Vettor, Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532), Ann Oncol, doi:10.1016/j.annonc.2020.04.479
Patel, Zhong, Liaw, Tremblay, Tsao et al., Does androgen deprivation therapy protect against severe complications from COVID-19?, Ann Oncol, doi:10.1016/j.annonc.2020.06.023
Qu, Gu, Wang, Metabolomic profiling to evaluate the efficacy of proxalutamide, a novel androgen receptor antagonist, in prostate cancer cells, Invest New Drugs, doi:10.1007/s10637-020-00901-w
Ranzani, Bastos, Gelli, Marchesi, Baião et al., Characterisation of the first 250,000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data, Lancet Respir Med, doi:10.1016/S2213-2600(20)30560-9
Rocco, Silva, Cruz, Early use of nitazoxanide in mild COVID-19 disease: randomised, placebo-controlled trial, Eur Respir J, doi:10.1183/13993003.03725-2020
Salazar Arenas, Del Carpio-Toia, Galdos, Rodriguez-Morales, Alopecia and severity of COVID-19: a cross-sectional study in Peru, Infez Med
Santos, Sayegh, Groehs, Testosterone deficiency increases hospital readmission and mortality rates in male patients with heart failure, Arq Bras Cardiol, doi:10.5935/abc.20150078
Stasi, Rastrelli, Inglese, Higher testosterone is associated with increased inflammatory markers in women with SARS-CoV-2 pneumonia: preliminary results from an observational study (PREPRINT), J Endocrinol Invest, doi:10.1007/s40618-021-01682-6
Subramanian, Anand, Adderley, Increased COVID-19 infections in women with polycystic ovary syndrome: a population-based study, Eur J Endocrinol, doi:10.1530/EJE-20-1163
Taira, Merrick, Galbreath, Butler, Adamovich, Impact of androgen deprivation therapy on overall mortality in prostate brachytherapy patients with low pretreatment testosterone levels, Am J Clin Oncol, doi:10.1097/COC.0000000000000340
Tong, Chen, Wu, Proxalutamide (GT0918), a potent androgen receptor pathway inhibitor, Cancer Res, doi:10.1158/1538-7445.AM2014-614
Velavan, Pallerla, Rüter, Kremsner, Krishna et al., Host genetic factors determining COVID-19 susceptibility and severity, EBioMedicine, doi:10.1016/j.ebiom.2021.103629
Wambier, Mccoy, Goren, Male balding as a major risk factor for severe COVID-19: a possible role for targeting androgens and transmembrane protease serine 2 to protect vulnerable individuals, J Am Acad Dermatol, doi:10.1016/j.jaad.2020.09.015
Wambier, Vaño-Galván, Mccoy, Androgenetic alopecia present in the majority of patients hospitalized with COVID-19: the "Gabrin sign, J Am Acad Dermatol, doi:10.1016/j.jaad.2020.05.079
Wang, Mannan, Xiao, Characterization of SARS-CoV-2 and host entry factors distribution in a COVID-19 autopsy series, Commun Med, doi:10.1038/s43856-021-00025-z
Zhang, Xiang, Huo, Zhou, Jiang et al., Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy, Signal Transduct Target Ther, doi:10.1038/s41392-021-00653-w
Zhao, Lou, Cao, An early assessment of a case fatality risk associated with P.1 SARS-CoV-2 lineage in Brazil: an ecological study, J Travel Med, doi:10.1093/jtm/taab078
Zimerman, Ferrareze, Cadegiani, Comparative genomics and characterization of SARS-CoV-2 P.1 (Gamma) Variant of Concern (VOC) from Amazonas, doi:10.1101/2021.10.30.21265694
Zimerman, Fonseca, Correia, Proxalutamide reduction of mortality rate in hospitalized COVID-19 patients depends on treatment duration -an exploratory analysis of the Proxa-Rescue AndroCoV trial, medRxiv, doi:10.1101/2021.06.28.21259661
DOI record:
{
"DOI": "10.7759/cureus.20691",
"ISSN": [
"2168-8184"
],
"URL": "http://dx.doi.org/10.7759/cureus.20691",
"author": [
{
"affiliation": [],
"family": "Cadegiani",
"given": "Flavio A",
"sequence": "first"
},
{
"affiliation": [],
"family": "Zimerman",
"given": "Ricardo A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fonseca",
"given": "Daniel N",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Correia",
"given": "Michael N",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Muller",
"given": "Marcio P",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bet",
"given": "Diego Leonardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Slaviero",
"given": "Marcio Rafael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zardo",
"given": "Ivan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Benites",
"given": "Paulo Roberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barros",
"given": "Renan N",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paulain",
"given": "Raysa W",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Onety",
"given": "Dirce C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Israel",
"given": "Karla Cristina P",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gustavo Wambier",
"given": "Carlos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goren",
"given": "Andy",
"sequence": "additional"
}
],
"container-title": [
"Cureus"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
12,
25
]
],
"date-time": "2021-12-25T21:07:21Z",
"timestamp": 1640466441000
},
"deposited": {
"date-parts": [
[
2021,
12,
25
]
],
"date-time": "2021-12-25T21:07:26Z",
"timestamp": 1640466446000
},
"indexed": {
"date-parts": [
[
2021,
12,
26
]
],
"date-time": "2021-12-26T05:34:46Z",
"timestamp": 1640496886225
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "2168-8184"
}
],
"issued": {
"date-parts": [
[
2021,
12,
25
]
]
},
"language": "en",
"link": [
{
"URL": "https://www.cureus.com/articles/80171-final-results-of-a-randomized-placebo-controlled-two-arm-parallel-clinical-trial-of-proxalutamide-for-hospitalized-covid-19-patients-a-multiregional-joint-analysis-of-the-proxa-rescue-androcov-trial",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "4492",
"original-title": [],
"prefix": "10.7759",
"published": {
"date-parts": [
[
2021,
12,
25
]
]
},
"published-print": {
"date-parts": [
[
2021,
12,
25
]
]
},
"publisher": "Cureus, Inc.",
"reference": [
{
"DOI": "10.1186/s13293-020-00304-9",
"article-title": "Impact of sex and gender on COVID-19 outcomes in Europe",
"author": "Gebhard C",
"doi-asserted-by": "publisher",
"journal-title": "Biol Sex Differ",
"key": "ref1",
"unstructured": "Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020, 11:29. 10.1186/s13293-020-00304-9",
"volume": "11",
"year": "2020"
},
{
"article-title": "COVID-19 clinical features",
"author": "McIntosh K",
"key": "ref2",
"unstructured": "McIntosh K. COVID-19 clinical features. UpToDate. Post TW (ed): UpToDate, Waltham, MA; 2021.",
"year": "2021"
},
{
"DOI": "10.1016/j.jaad.2020.07.062",
"article-title": "Male balding is a major risk factor for severe COVID-19",
"author": "Lee J",
"doi-asserted-by": "publisher",
"journal-title": "J Am Acad Dermatol",
"key": "ref3",
"unstructured": "Lee J, Yousaf A, Fang W, Kolodney MS. Male balding is a major risk factor for severe COVID-19. J Am Acad Dermatol. 2020, 83:353-354. 10.1016/j.jaad.2020.07.062",
"volume": "83",
"year": "2020"
},
{
"DOI": "10.1016/j.jaad.2020.05.079",
"article-title": "Androgenetic alopecia present in the majority of patients hospitalized with COVID-19: the \"Gabrin sign\"",
"author": "Wambier CG",
"doi-asserted-by": "publisher",
"journal-title": "J Am Acad Dermatol",
"key": "ref4",
"unstructured": "Wambier CG, Vaño-Galván S, McCoy J, et al.. Androgenetic alopecia present in the majority of patients hospitalized with COVID-19: the \"Gabrin sign\". J Am Acad Dermatol. 2020, 83:680-682. 10.1016/j.jaad.2020.05.079",
"volume": "83",
"year": "2020"
},
{
"article-title": "Alopecia and severity of COVID-19: a cross-sectional study in Peru",
"author": "Salazar Arenas MÁ",
"journal-title": "Infez Med",
"key": "ref5",
"unstructured": "Salazar Arenas MÁ, Muñoz Del Carpio-Toia A, Aybar Galdos J, Rodriguez-Morales AJ. Alopecia and severity of COVID-19: a cross-sectional study in Peru. Infez Med. 2021, 29:37-45.",
"volume": "29",
"year": "2021"
},
{
"DOI": "10.1016/j.jaad.2020.09.015",
"article-title": "Male balding as a major risk factor for severe COVID-19: a possible role for targeting androgens and transmembrane protease serine 2 to protect vulnerable individuals",
"author": "Wambier CG",
"doi-asserted-by": "publisher",
"journal-title": "J Am Acad Dermatol",
"key": "ref6",
"unstructured": "Wambier CG, McCoy J, Goren A. Male balding as a major risk factor for severe COVID-19: a possible role for targeting androgens and transmembrane protease serine 2 to protect vulnerable individuals. J Am Acad Dermatol. 2020, 83:401-402. 10.1016/j.jaad.2020.09.015",
"volume": "83",
"year": "2020"
},
{
"DOI": "10.1136/bcr-2021-241572",
"article-title": "Potential risk for developing severe COVID-19 disease among anabolic steroid users",
"author": "Cadegiani F",
"doi-asserted-by": "publisher",
"journal-title": "BMJ Case Rep",
"key": "ref7",
"unstructured": "Cadegiani F, Lin EM, Goren A, Wambier CG. Potential risk for developing severe COVID-19 disease among anabolic steroid users. BMJ Case Rep. 2021, 14:e241572. 10.1136/bcr-2021-241572",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1111/jdv.16956",
"article-title": "Androgen receptor genetic variant predicts COVID-19 disease severity: a prospective longitudinal study of hospitalized COVID-19 male patients",
"author": "McCoy J",
"doi-asserted-by": "publisher",
"journal-title": "J Eur Acad Dermatol Venereol",
"key": "ref8",
"unstructured": "McCoy J, Wambier CG, Herrera S, et al.. Androgen receptor genetic variant predicts COVID-19 disease severity: a prospective longitudinal study of hospitalized COVID-19 male patients. J Eur Acad Dermatol Venereol. 2021, 35:15-17. 10.1111/jdv.16956",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1016/j.ebiom.2021.103629",
"article-title": "Host genetic factors determining COVID-19 susceptibility and severity",
"author": "Velavan TP",
"doi-asserted-by": "publisher",
"journal-title": "EBioMedicine",
"key": "ref9",
"unstructured": "Velavan TP, Pallerla SR, Rüter J, Augustin Y, Kremsner PG, Krishna S, Meyer CG. Host genetic factors determining COVID-19 susceptibility and severity. EBioMedicine. 2021, 72:103629. 10.1016/j.ebiom.2021.103629",
"volume": "72",
"year": "2021"
},
{
"DOI": "10.1530/EJE-20-1163",
"article-title": "Increased COVID-19 infections in women with polycystic ovary syndrome: a population-based study",
"author": "Subramanian A",
"doi-asserted-by": "publisher",
"journal-title": "Eur J Endocrinol",
"key": "ref10",
"unstructured": "Subramanian A, Anand A, Adderley NJ, et al.. Increased COVID-19 infections in women with polycystic ovary syndrome: a population-based study. Eur J Endocrinol. 2021, 184:637-645. 10.1530/EJE-20-1163",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1111/jdv.17004",
"article-title": "Clinical symptoms of hyperandrogenic women diagnosed with COVID-19",
"author": "Cadegiani FA",
"doi-asserted-by": "publisher",
"journal-title": "J Eur Acad Dermatol Venereol",
"key": "ref11",
"unstructured": "Cadegiani FA, Lim RK, Goren A, et al.. Clinical symptoms of hyperandrogenic women diagnosed with COVID-19. J Eur Acad Dermatol Venereol. 2021, 35:101-104. 10.1111/jdv.17004",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1111/jdv.16953",
"article-title": "Anti-androgens may protect against severe COVID-19 outcomes: results from a prospective cohort study of 77 hospitalized men",
"author": "Goren A",
"doi-asserted-by": "publisher",
"journal-title": "J Eur Acad Dermatol Venereol",
"key": "ref12",
"unstructured": "Goren A, Wambier CG, Herrera S, et al.. Anti-androgens may protect against severe COVID-19 outcomes: results from a prospective cohort study of 77 hospitalized men. J Eur Acad Dermatol Venereol. 2021, 35:13-15. 10.1111/jdv.16953",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1111/jdv.17021",
"article-title": "5-alpha-reductase inhibitors are associated with reduced frequency of COVID-19 symptoms in males with androgenetic alopecia",
"author": "McCoy J",
"doi-asserted-by": "publisher",
"journal-title": "J Eur Acad Dermatol Venereol",
"key": "ref13",
"unstructured": "McCoy J, Cadegiani FA, Wambier CG, et al.. 5-alpha-reductase inhibitors are associated with reduced frequency of COVID-19 symptoms in males with androgenetic alopecia. J Eur Acad Dermatol Venereol. 2021, 35:243-246. 10.1111/jdv.17021",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1016/j.annonc.2020.06.023",
"article-title": "Does androgen deprivation therapy protect against severe complications from COVID-19?",
"author": "Patel VG",
"doi-asserted-by": "publisher",
"journal-title": "Ann Oncol",
"key": "ref14",
"unstructured": "Patel VG, Zhong X, Liaw B, Tremblay D, Tsao CK, Galsky MD, Oh WK. Does androgen deprivation therapy protect against severe complications from COVID-19?. Ann Oncol. 2020, 31:1419-1420. 10.1016/j.annonc.2020.06.023",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1016/j.annonc.2020.04.479",
"article-title": "Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532)",
"author": "Montopoli M",
"doi-asserted-by": "publisher",
"journal-title": "Ann Oncol",
"key": "ref15",
"unstructured": "Montopoli M, Zumerle S, Vettor R, et al.. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Ann Oncol. 2020, 31:1040-1045. 10.1016/j.annonc.2020.04.479",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1530/ERC-20-0165",
"article-title": "COVID-19 and androgen-targeted therapy for prostate cancer patients",
"author": "Bhowmick NA",
"doi-asserted-by": "publisher",
"journal-title": "Endocr Relat Cancer",
"key": "ref16",
"unstructured": "Bhowmick NA, Oft J, Dorff T, et al.. COVID-19 and androgen-targeted therapy for prostate cancer patients. Endocr Relat Cancer. 2020, 27:281-292. 10.1530/ERC-20-0165",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1101/2021.05.10.21255146",
"article-title": "A population-level analysis of the protective effects of androgen deprivation therapy against COVID-19 disease incidence and severity (PREPRINT)",
"author": "Lee KM",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "ref17",
"unstructured": "Lee KM, Heberer K, Gao A, et al.. A population-level analysis of the protective effects of androgen deprivation therapy against COVID-19 disease incidence and severity (PREPRINT). medRxiv. 2021, 10.1101/2021.05.10.21255146",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor",
"author": "Hoffmann M",
"doi-asserted-by": "publisher",
"journal-title": "Cell",
"key": "ref18",
"unstructured": "Hoffmann M, Kleine-Weber H, Schroeder S, et al.. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020, 181:271-280. 10.1016/j.cell.2020.02.052",
"volume": "181",
"year": "2020"
},
{
"article-title": "Catalytic cleavage of the androgen-regulated TMPRSS2 protease results in its secretion by prostate and prostate cancer epithelia",
"author": "Afar DE",
"journal-title": "Cancer Res",
"key": "ref19",
"unstructured": "Afar DE, Vivanco I, Hubert RS, et al.. Catalytic cleavage of the androgen-regulated TMPRSS2 protease results in its secretion by prostate and prostate cancer epithelia. Cancer Res. 2001, 61:1686-1692.",
"volume": "61",
"year": "2001"
},
{
"DOI": "10.3390/pathogens10080988",
"article-title": "Predominance of the SARS-CoV-2 lineage P.1 and its sublineage P.1.2 in patients from the metropolitan region of Porto Alegre, Southern Brazil in March 2021",
"author": "Franceschi VB",
"doi-asserted-by": "publisher",
"journal-title": "Pathogens",
"key": "ref20",
"unstructured": "Franceschi VB, Caldana GD, Perin C, et al.. Predominance of the SARS-CoV-2 lineage P.1 and its sublineage P.1.2 in patients from the metropolitan region of Porto Alegre, Southern Brazil in March 2021. Pathogens. 2021, 10:988. 10.3390/pathogens10080988",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1038/s41392-021-00653-w",
"article-title": "Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy",
"author": "Zhang Q",
"doi-asserted-by": "publisher",
"journal-title": "Signal Transduct Target Ther",
"key": "ref21",
"unstructured": "Zhang Q, Xiang R, Huo S, Zhou Y, Jiang S, Wang Q, Yu F. Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy. Signal Transduct Target Ther. 2021, 6:233. 10.1038/s41392-021-00653-w",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1186/s12902-020-00626-0",
"article-title": "Repurposing existing drugs for COVID-19: an endocrinology perspective",
"author": "Cadegiani FA",
"doi-asserted-by": "publisher",
"journal-title": "BMC Endocr Disord",
"key": "ref22",
"unstructured": "Cadegiani FA. Repurposing existing drugs for COVID-19: an endocrinology perspective. BMC Endocr Disord. 2020, 20:149. 10.1186/s12902-020-00626-0",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.3389/fmed.2020.00453",
"article-title": "Spironolactone: an anti-androgenic and anti-hypertensive drug that may provide protection against the novel coronavirus (SARS-CoV-2) induced acute respiratory distress syndrome (ARDS) in COVID-19",
"author": "Cadegiani FA",
"doi-asserted-by": "publisher",
"journal-title": "Front Med (Lausanne)",
"key": "ref23",
"unstructured": "Cadegiani FA, Wambier CG, Goren A. Spironolactone: an anti-androgenic and anti-hypertensive drug that may provide protection against the novel coronavirus (SARS-CoV-2) induced acute respiratory distress syndrome (ARDS) in COVID-19. Front Med (Lausanne). 2020, 7:453. 10.3389/fmed.2020.00453",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.7759/cureus.13047",
"article-title": "Early antiandrogen therapy with dutasteride reduces viral shedding, inflammatory responses, and time-to-remission in males with COVID-19: a randomized, double-blind, placebo-controlled interventional trial (EAT-DUTA AndroCoV Trial - Biochemical)",
"author": "Cadegiani FA",
"doi-asserted-by": "publisher",
"journal-title": "Cureus",
"key": "ref24",
"unstructured": "Cadegiani FA, McCoy J, Gustavo Wambier C, Goren A. Early antiandrogen therapy with dutasteride reduces viral shedding, inflammatory responses, and time-to-remission in males with COVID-19: a randomized, double-blind, placebo-controlled interventional trial (EAT-DUTA AndroCoV Trial - Biochemical). Cureus. 2021, 13:e13047. 10.7759/cureus.13047",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1007/s10637-020-00901-w",
"article-title": "Metabolomic profiling to evaluate the efficacy of proxalutamide, a novel androgen receptor antagonist, in prostate cancer cells",
"author": "Qu F",
"doi-asserted-by": "publisher",
"journal-title": "Invest New Drugs",
"key": "ref25",
"unstructured": "Qu F, Gu Y, Wang Q, et al.. Metabolomic profiling to evaluate the efficacy of proxalutamide, a novel androgen receptor antagonist, in prostate cancer cells. Invest New Drugs. 2020, 38:1292-1302. 10.1007/s10637-020-00901-w",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.3389/fmed.2021.668698",
"article-title": "Proxalutamide reduces the rate of hospitalization for COVID-19 male outpatients: a randomized double-blinded placebo-controlled trial",
"author": "McCoy J",
"doi-asserted-by": "publisher",
"journal-title": "Front Med (Lausanne)",
"key": "ref26",
"unstructured": "McCoy J, Goren A, Cadegiani FA, et al.. Proxalutamide reduces the rate of hospitalization for COVID-19 male outpatients: a randomized double-blinded placebo-controlled trial. Front Med (Lausanne). 2021, 8:668698. 10.3389/fmed.2021.668698",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1101/2021.07.06.21260086",
"article-title": "Proxalutamide (GT0918) reduces the rate of hospitalization in mild-to-moderate COVID-19 female patients: a randomized double-blinded placebo-controlled two-arm parallel trial (PREPRINT)",
"author": "Cadegiani FA",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "ref27",
"unstructured": "Cadegiani FA, Zimerman RA, Fonseca DN, Correia MC, McCoy J, Wambier CG, Goren A. Proxalutamide (GT0918) reduces the rate of hospitalization in mild-to-moderate COVID-19 female patients: a randomized double-blinded placebo-controlled two-arm parallel trial (PREPRINT). medRxiv. 2021, 10.1101/2021.07.06.21260086",
"year": "2021"
},
{
"DOI": "10.7759/cureus.13492",
"article-title": "Proxalutamide significantly accelerates viral clearance and reduces time to clinical remission in patients with mild to moderate COVID-19: results from a randomized, double-blinded, placebo-controlled trial",
"author": "Cadegiani FA",
"doi-asserted-by": "publisher",
"journal-title": "Cureus",
"key": "ref28",
"unstructured": "Cadegiani FA, McCoy J, Gustavo Wambier C, et al.. Proxalutamide significantly accelerates viral clearance and reduces time to clinical remission in patients with mild to moderate COVID-19: results from a randomized, double-blinded, placebo-controlled trial. Cureus. 2021, 13:e13492. 10.7759/cureus.13492",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1101/2021.07.24.21261047",
"article-title": "Proxalutamide improves inflammatory, immunologic, and thrombogenic markers in mild-to-moderate COVID-19 males and females: an exploratory analysis of a randomized, double-blinded, placebo-controlled trial early antiandrogen therapy (EAT) with proxalutamide (The EAT-Proxa Biochemical AndroCoV-Trial) (PREPRINT)",
"author": "Cadegiani FA",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "ref29",
"unstructured": "Cadegiani FA, Goren A, Wambier CG, Zimerman RA. Proxalutamide improves inflammatory, immunologic, and thrombogenic markers in mild-to-moderate COVID-19 males and females: an exploratory analysis of a randomized, double-blinded, placebo-controlled trial early antiandrogen therapy (EAT) with proxalutamide (The EAT-Proxa Biochemical AndroCoV-Trial) (PREPRINT). medRxiv. 2021, 10.1101/2021.07.24.21261047",
"year": "2021"
},
{
"DOI": "10.1101/2021.06.22.21259318",
"article-title": "Efficacy of proxalutamide in hospitalized COVID-19 patients: a randomized, double-blind, placebo-controlled, parallel-design clinical trial (PREPRINT)",
"author": "Cadegiani FA",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "ref30",
"unstructured": "Cadegiani FA, Fonseca DN, McCoy J, et al.. Efficacy of proxalutamide in hospitalized COVID-19 patients: a randomized, double-blind, placebo-controlled, parallel-design clinical trial (PREPRINT). medRxiv. 2021, 10.1101/2021.06.22.21259318",
"year": "2021"
},
{
"article-title": "Proxalutamide Treatment for Hospitalized COVID-19 Patients in Southern Brazil: The South Arm of a Randomized, Double-Blind, Placebo-Controlled, Parallel Clinical Trial - The South Proxa-Rescue AndroCoV Trial (PREPRINT)",
"author": "Flávio C",
"key": "ref31",
"unstructured": "Flávio C, Zimerman RA, Goren A, Wambier CG. Proxalutamide Treatment for Hospitalized COVID-19 Patients in Southern Brazil: The South Arm of a Randomized, Double-Blind, Placebo-Controlled, Parallel Clinical Trial - The South Proxa-Rescue AndroCoV Trial (PREPRINT). 2021.",
"year": "2021"
},
{
"DOI": "10.1101/2021.06.28.21259661",
"article-title": "Proxalutamide reduction of mortality rate in hospitalized COVID-19 patients depends on treatment duration - an exploratory analysis of the Proxa-Rescue AndroCoV trial",
"author": "Zimerman RA",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "ref32",
"unstructured": "Zimerman RA, Fonseca DN, Correia MN, et al.. Proxalutamide reduction of mortality rate in hospitalized COVID-19 patients depends on treatment duration - an exploratory analysis of the Proxa-Rescue AndroCoV trial. medRxiv. 2021, 10.1101/2021.06.28.21259661",
"year": "2021"
},
{
"DOI": "10.1101/2021.07.01.21259656",
"article-title": "Proxalutamide improves lung injury in hospitalized COVID-19 patients - an analysis of the radiological findings of the Proxa-Rescue AndroCoV trial (PREPRINT)",
"author": "Cadegiani FA",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "ref33",
"unstructured": "Cadegiani FA, Fonseca DN, Correia MN, et al.. Proxalutamide improves lung injury in hospitalized COVID-19 patients - an analysis of the radiological findings of the Proxa-Rescue AndroCoV trial (PREPRINT). medRxiv. 2021, 10.1101/2021.07.01.21259656",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(20)30560-9",
"article-title": "Characterisation of the first 250,000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data",
"author": "Ranzani OT",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Respir Med",
"key": "ref34",
"unstructured": "Ranzani OT, Bastos LS, Gelli JG, Marchesi JF, Baião F, Hamacher S, Bozza FA. Characterisation of the first 250,000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data. Lancet Respir Med. 2021, 9:407-418. 10.1016/S2213-2600(20)30560-9",
"volume": "9",
"year": "2021"
},
{
"key": "ref35",
"unstructured": "Demographic characteristics of regions in Brazil. (2021). Accessed. December 5, 2021: http://www.ibge.gov.br."
},
{
"key": "ref36",
"unstructured": "SARS-CoV-2 variant classifications and definitions. (2021). Accessed. December 25, 2021: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html."
},
{
"DOI": "10.1101/2021.04.13.21255281",
"article-title": "The increase in the risk of severity and fatality rate of covid-19 in southern Brazil after the emergence of the Variant of Concern (VOC) SARS-CoV-2 P.1 was greater among young adults without pre-existing risk conditions (PREPRINT)",
"author": "Freitas ARR",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "ref37",
"unstructured": "Freitas ARR, Lemos DRQ, Beckedorff OA, Cavalcanti LPG, Siqueira AM, de Mello RCS, Barros ENC. The increase in the risk of severity and fatality rate of covid-19 in southern Brazil after the emergence of the Variant of Concern (VOC) SARS-CoV-2 P.1 was greater among young adults without pre-existing risk conditions (PREPRINT). medRxiv. 2021, 10.1101/2021.04.13.21255281",
"year": "2021"
},
{
"DOI": "10.1093/jtm/taab078",
"article-title": "An early assessment of a case fatality risk associated with P.1 SARS-CoV-2 lineage in Brazil: an ecological study",
"author": "Zhao S",
"doi-asserted-by": "publisher",
"journal-title": "J Travel Med",
"key": "ref38",
"unstructured": "Zhao S, Lou J, Cao L, et al.. An early assessment of a case fatality risk associated with P.1 SARS-CoV-2 lineage in Brazil: an ecological study. J Travel Med. 2021, 28:taab078. 10.1093/jtm/taab078",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1101/2021.10.30.21265694",
"article-title": "Comparative genomics and characterization of SARS-CoV-2 P.1 (Gamma) Variant of Concern (VOC) from Amazonas, Brazil (PREPRINT)",
"author": "Zimerman RA",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "ref39",
"unstructured": "Zimerman RA, Ferrareze PAG, Cadegiani FA, et al.. Comparative genomics and characterization of SARS-CoV-2 P.1 (Gamma) Variant of Concern (VOC) from Amazonas, Brazil (PREPRINT). medRxiv. 2021, 10.1101/2021.10.30.21265694",
"year": "2021"
},
{
"DOI": "10.1007/s40618-021-01682-6",
"article-title": "Higher testosterone is associated with increased inflammatory markers in women with SARS-CoV-2 pneumonia: preliminary results from an observational study (PREPRINT)",
"author": "Di Stasi V",
"doi-asserted-by": "publisher",
"journal-title": "J Endocrinol Invest",
"key": "ref40",
"unstructured": "Di Stasi V, Rastrelli G, Inglese F, et al.. Higher testosterone is associated with increased inflammatory markers in women with SARS-CoV-2 pneumonia: preliminary results from an observational study (PREPRINT). J Endocrinol Invest. 2021, 10.1007/s40618-021-01682-6",
"year": "2021"
},
{
"DOI": "10.3390/v13112209",
"article-title": "SARS-CoV-2 spike protein S1-mediated endothelial injury and pro-inflammatory state is amplified by dihydrotestosterone and prevented by mineralocorticoid antagonism",
"author": "Kumar N",
"doi-asserted-by": "publisher",
"journal-title": "Viruses",
"key": "ref41",
"unstructured": "Kumar N, Zuo Y, Yalavarthi S, et al.. SARS-CoV-2 spike protein S1-mediated endothelial injury and pro-inflammatory state is amplified by dihydrotestosterone and prevented by mineralocorticoid antagonism. Viruses. 2021, 13:2209. 10.3390/v13112209",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1038/s43856-021-00025-z",
"article-title": "Characterization of SARS-CoV-2 and host entry factors distribution in a COVID-19 autopsy series",
"author": "Wang XM",
"doi-asserted-by": "publisher",
"journal-title": "Commun Med",
"key": "ref42",
"unstructured": "Wang XM, Mannan R, Xiao L, et al.. Characterization of SARS-CoV-2 and host entry factors distribution in a COVID-19 autopsy series. Commun Med. 2021, 1:24. 10.1038/s43856-021-00025-z",
"volume": "1",
"year": "2021"
},
{
"DOI": "10.1038/s41467-021-24342-y",
"article-title": "The antiandrogen enzalutamide downregulates TMPRSS2 and reduces cellular entry of SARS-CoV-2 in human lung cells",
"author": "Leach DA",
"doi-asserted-by": "publisher",
"journal-title": "Nat Commun",
"key": "ref43",
"unstructured": "Leach DA, Mohr A, Giotis ES, et al.. The antiandrogen enzalutamide downregulates TMPRSS2 and reduces cellular entry of SARS-CoV-2 in human lung cells. Nat Commun. 2021, 12:4068. 10.1038/s41467-021-24342-y",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.chest.2021.02.024",
"article-title": "Progesterone in addition to standard of care vs standard of care alone in the treatment of men hospitalized with moderate to severe COVID-19: a randomized, controlled pilot trial",
"author": "Ghandehari S",
"doi-asserted-by": "publisher",
"journal-title": "Chest",
"key": "ref44",
"unstructured": "Ghandehari S, Matusov Y, Pepkowitz S, et al.. Progesterone in addition to standard of care vs standard of care alone in the treatment of men hospitalized with moderate to severe COVID-19: a randomized, controlled pilot trial. Chest. 2021, 160:74-84. 10.1016/j.chest.2021.02.024",
"volume": "160",
"year": "2021"
},
{
"DOI": "10.6061/clinics/2020/e1980",
"article-title": "17β-Estradiol, a potential ally to alleviate SARS-CoV-2 infection",
"author": "Breithaupt-Faloppa AC",
"doi-asserted-by": "publisher",
"journal-title": "Clinics (Sao Paulo)",
"key": "ref45",
"unstructured": "Breithaupt-Faloppa AC, de Jesus Correia C, Prado CM, Stilhano RS, Ureshino RP, Moreira LF. 17β-Estradiol, a potential ally to alleviate SARS-CoV-2 infection. Clinics (Sao Paulo). 2020, 75:e1980. 10.6061/clinics/2020/e1980",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1101/2021.02.16.21251853",
"article-title": "Mortality in COVID-19 amongst women on hormone replacement therapy or combined oral contraception: a cohort study (PREPRINT)",
"author": "Dambha-Miller H",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "ref46",
"unstructured": "Dambha-Miller H, Hinton W, Joy M, Feher M, de Lusignan S. Mortality in COVID-19 amongst women on hormone replacement therapy or combined oral contraception: a cohort study (PREPRINT). medRxiv. 2021, 10.1101/2021.02.16.21251853",
"year": "2021"
},
{
"DOI": "10.1080/0092623X.2021.2000535",
"article-title": "TransCOVID: does gender-affirming hormone therapy play a role in contracting COVID-19?",
"author": "Durcan E",
"doi-asserted-by": "publisher",
"journal-title": "J Sex Marital Ther",
"key": "ref47",
"unstructured": "Durcan E, Turan S, Bircan BE, et al.. TransCOVID: does gender-affirming hormone therapy play a role in contracting COVID-19?. J Sex Marital Ther. 2021, 1-12. 10.1080/0092623X.2021.2000535",
"year": "2021"
},
{
"DOI": "10.1016/j.nmni.2021.100915",
"article-title": "Early COVID-19 therapy with azithromycin plus nitazoxanide, ivermectin or hydroxychloroquine in outpatient settings significantly improved COVID-19 outcomes compared to known outcomes in untreated patients",
"author": "Cadegiani FA",
"doi-asserted-by": "publisher",
"journal-title": "New Microbes New Infect",
"key": "ref48",
"unstructured": "Cadegiani FA, Goren A, Wambier CG, McCoy J. Early COVID-19 therapy with azithromycin plus nitazoxanide, ivermectin or hydroxychloroquine in outpatient settings significantly improved COVID-19 outcomes compared to known outcomes in untreated patients. New Microbes New Infect. 2021, 43:100915. 10.1016/j.nmni.2021.100915",
"volume": "43",
"year": "2021"
},
{
"DOI": "10.1183/13993003.03725-2020",
"article-title": "Early use of nitazoxanide in mild COVID-19 disease: randomised, placebo-controlled trial",
"author": "Rocco PR",
"doi-asserted-by": "publisher",
"journal-title": "Eur Respir J",
"key": "ref49",
"unstructured": "Rocco PR, Silva PL, Cruz FF, et al.. Early use of nitazoxanide in mild COVID-19 disease: randomised, placebo-controlled trial. Eur Respir J. 2021, 58:2003725. 10.1183/13993003.03725-2020",
"volume": "58",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2021.100981",
"article-title": "Nitazoxanide superiority to placebo to treat moderate COVID-19 - A Pilot prove of concept randomized double-blind clinical trial",
"author": "Blum VF",
"doi-asserted-by": "publisher",
"journal-title": "EClinicalMedicine",
"key": "ref50",
"unstructured": "Blum VF, Cimerman S, Hunter JR, et al.. Nitazoxanide superiority to placebo to treat moderate COVID-19 - A Pilot prove of concept randomized double-blind clinical trial. EClinicalMedicine. 2021, 37:100981. 10.1016/j.eclinm.2021.100981",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1136/bmj.n967",
"article-title": "Drug treatments for covid-19: living systematic review and network meta-analysis",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "ref51",
"unstructured": "Drug treatments for covid-19. living systematic review and network meta-analysis. BMJ. 2021, 373:n967. 10.1136/bmj.n967",
"volume": "373",
"year": "2021"
},
{
"article-title": "Drug cocktail restores partial immunity",
"journal-title": "AIDS Alert",
"key": "ref52",
"unstructured": "Drug cocktail restores partial immunity. AIDS Alert. 1997, 12.48.",
"volume": "12",
"year": "1997"
},
{
"article-title": "Alarmed researchers push for more aggressive treatment and monitoring",
"journal-title": "AIDS Alert",
"key": "ref53",
"unstructured": "Alarmed researchers push for more aggressive treatment and monitoring. AIDS Alert. 1997, 12.109-111.",
"volume": "12",
"year": "1997"
},
{
"DOI": "10.1038/s41573-019-0037-0",
"article-title": "Therapeutic strategies for hepatitis B virus infection: towards a cure",
"author": "Fanning GC",
"doi-asserted-by": "publisher",
"journal-title": "Nat Rev Drug Discov",
"key": "ref54",
"unstructured": "Fanning GC, Zoulim F, Hou J, Bertoletti A. Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat Rev Drug Discov. 2019, 18:827-844. 10.1038/s41573-019-0037-0",
"volume": "18",
"year": "2019"
},
{
"DOI": "10.1101/cshperspect.a037069",
"article-title": "Hepatitis C virus: 30 years after its discovery",
"author": "Houghton M",
"doi-asserted-by": "publisher",
"journal-title": "Cold Spring Harb Perspect Med",
"key": "ref55",
"unstructured": "Houghton M. Hepatitis C virus: 30 years after its discovery. Cold Spring Harb Perspect Med. 2019, 9:037069. 10.1101/cshperspect.a037069",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1097/COC.0000000000000340",
"article-title": "Impact of androgen deprivation therapy on overall mortality in prostate brachytherapy patients with low pretreatment testosterone levels",
"author": "Taira AV",
"doi-asserted-by": "publisher",
"journal-title": "Am J Clin Oncol",
"key": "ref56",
"unstructured": "Taira AV, Merrick GS, Galbreath RW, Butler WM, Adamovich E. Impact of androgen deprivation therapy on overall mortality in prostate brachytherapy patients with low pretreatment testosterone levels. Am J Clin Oncol. 2018, 41:667-673. 10.1097/COC.0000000000000340",
"volume": "41",
"year": "2018"
},
{
"DOI": "10.1001/jamanetworkopen.2021.11398",
"article-title": "Association of circulating sex hormones with inflammation and disease severity in patients with COVID-19",
"author": "Dhindsa S",
"doi-asserted-by": "publisher",
"journal-title": "JAMA Netw Open",
"key": "ref57",
"unstructured": "Dhindsa S, Zhang N, McPhaul MJ, et al.. Association of circulating sex hormones with inflammation and disease severity in patients with COVID-19. JAMA Netw Open. 2021, 4:e2111398. 10.1001/jamanetworkopen.2021.11398",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.5935/abc.20150078",
"article-title": "Testosterone deficiency increases hospital readmission and mortality rates in male patients with heart failure",
"author": "Santos MR",
"doi-asserted-by": "publisher",
"journal-title": "Arq Bras Cardiol",
"key": "ref58",
"unstructured": "Santos MR, Sayegh AL, Groehs RV, et al.. Testosterone deficiency increases hospital readmission and mortality rates in male patients with heart failure. Arq Bras Cardiol. 2015, 105:256-264. 10.5935/abc.20150078",
"volume": "105",
"year": "2015"
},
{
"DOI": "10.1016/j.clgc.2020.02.005",
"article-title": "A systematic review and network meta-analysis of novel androgen receptor inhibitors in non-metastatic castration-resistant prostate cancer",
"author": "Hird AE",
"doi-asserted-by": "publisher",
"journal-title": "Clin Genitourin Cancer",
"key": "ref59",
"unstructured": "Hird AE, Magee DE, Bhindi B, et al.. A systematic review and network meta-analysis of novel androgen receptor inhibitors in non-metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2020, 18:343-350. 10.1016/j.clgc.2020.02.005",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1158/1538-7445.AM2014-614",
"article-title": "Proxalutamide (GT0918), a potent androgen receptor pathway inhibitor",
"author": "Tong Y",
"doi-asserted-by": "publisher",
"journal-title": "Cancer Res",
"key": "ref60",
"unstructured": "Tong Y, Chen C, Wu J, et al.. Proxalutamide (GT0918), a potent androgen receptor pathway inhibitor. Cancer Res. 2014, 74:614. 10.1158/1538-7445.AM2014-614",
"volume": "74",
"year": "2014"
},
{
"key": "ref61",
"unstructured": "The safety and tolerability of proxalutamide (GT0918) in subjects with metastatic castrate resistant prostate cancer. (2021). Accessed. December 5, 2021: https://clinicaltrials.gov/ct2/show/NCT03899467."
}
],
"reference-count": 61,
"references-count": 61,
"relation": {},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subject": [
"Aerospace Engineering"
],
"subtitle": [],
"title": [
"Final Results of a Randomized, Placebo-Controlled, Two-Arm, Parallel Clinical Trial of Proxalutamide for Hospitalized COVID-19 Patients: A Multiregional, Joint Analysis of the Proxa-Rescue AndroCoV Trial"
],
"type": "journal-article"
}
cadegiani10