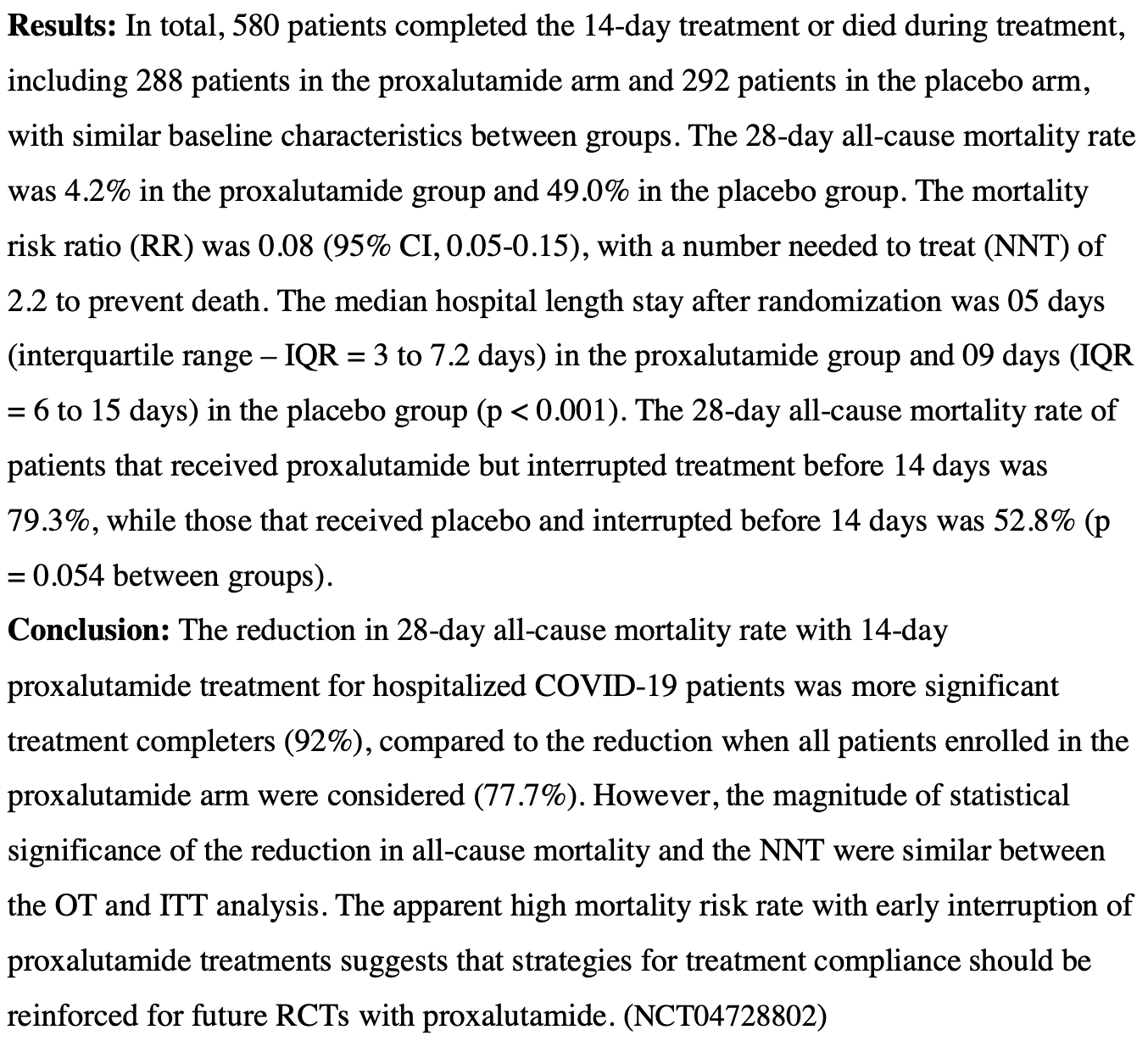
Proxalutamide (GT0918) Reduction of Mortality Rate in Hospitalized COVID-19 Patients Depends on Treatment Duration - an Exploratory Analysis of the Proxa-Rescue AndroCoV Trial
et al., medRxiv, doi:10.1101/2021.06.28.21259661, NCT04728802, Jul 2021
Analysis of treatment duration in the Proxa-Rescue AndroCoV trial showing significantly higher mortality among patients that interrupted treatment with proxalutamide. NCT04728802 (history).
Zimerman et al., 2 Jul 2021, preprint, 17 authors, trial NCT04728802 (history).
Proxalutamide Reduction of Mortality Rate in Hospitalized COVID-19 Patients Depends on Treatment Duration – an Exploratory Analysis of the Proxa-Rescue AndroCoV Trial
doi:10.1101/2021.06.28.21259661
Introduction: Proxalutamide, a second-generation non-steroidal antiandrogen (NSAA), primarily developed for castration-resistant prostate cancer, demonstrated reduction in 28-day mortality rate of 77.7% in hospitalized COVID-19 patients in a double-blind, placebo-controlled, two-arm randomized clinical trial (RCT), through intention-to-treat (ITT) analysis. We observed a high 28-day mortality rate of patients that did not complete the 14-day treatment with proxalutamide, compared to the placebo arm. These differences may raise hypotheses to explain the wide differences between ITT and ontreatment (OT) analysis in terms of efficacy. Despite the inherent limitations of OT analysis, we aimed to respond to unanswered questions regarding the drug efficacy when the 14-day treatment with proxalutamide was complete, and secondarily understand the causality relationship between treatment interruption and mortality rate. Methods: This is a post-hoc exploratory analysis of a double-blinded, randomized, placebo-controlled, prospective, multicentric, two-arm RCT of 300mg-daily 14-day proxalutamide therapy for hospitalized COVID-19 patients not requiring mechanical ventilation. OT population excluded patients that did not complete the full 14-day course of therapy or died from COVID-19 complications within 24 hours of randomization. The primary outcome was the 28-day COVID-19 mortality rate. Secondary outcomes included median hospital length, 14-day and 28-day alive hospital discharge rate and 28-day all-cause mortality rate of those who discontinued intervention. Results: In total, 580 patients completed the 14-day treatment or died during treatment, including 288 patients in the proxalutamide arm and 292 patients in the placebo arm, with similar baseline characteristics between groups. The 28-day COVID-19 mortality rate was 4.2% in the proxalutamide group and 49.0% in the placebo group. The mortality risk ratio (RR) was 0.08 (95% CI, 0.05-0.15), with a number needed to treat (NNT) of 2.2 to prevent death. The median hospital length stay after randomization was 5 days (interquartile range [IQR] = 3 to 7.2 days) in the proxalutamide group and 9 days (IQR = 6 to 15 days) in the placebo group (p <0.001). The 28-day all-cause mortality rate of patients that received proxalutamide but interrupted treatment before 14 days was 79.3%, while those that received placebo and interrupted before 14 days was 52.8% (p = 0.054 between groups).
Conclusion: The reduction in 28-day all-cause mortality rate with 14-day proxalutamide treatment for hospitalized COVID-19 patients was more significant while on treatment adhesion (92%), compared to the reduction when all patients enrolled in the proxalutamide arm were considered (77.7%). However, the magnitude of statistical significance of the reduction in all-cause mortality and the NNT were similar between the OT and ITT analysis. The apparent high mortality risk rate with early interruption of proxalutamide treatments..
References
Cadegiani, Lim, Goren, Mccoy, Situm et al., Clinical symptoms of hyperandrogenic women diagnosed with COVID-19, J Eur Acad Dermatol Venereol, doi:10.1111/jdv.17004
Cadegiani, Lin, Goren, Wambier, Potential risk for developing severe COVID-19 disease among anabolic steroid users, BMJ Case Rep, doi:10.1136/bcr-2021-241572
Cadegiani, Mccoy, Wambier, Goren, Early Antiandrogen Therapy With Dutasteride Reduces Viral Shedding, Inflammatory Responses, and Time-to-Remission in Males With COVID-19: A Randomized, Double-Blind, Placebo-Controlled Interventional Trial (EAT-DUTA AndroCoV Trial -Biochemical), Cureus, doi:10.7759/cureus.13047
Cadegiani, Mccoy, Wambier, Proxalutamide Significantly Accelerates Viral Clearance and Reduces Time to Clinical Remission in Patients with Mild to Moderate COVID-19: Results from a Randomized, Double-Blinded, Placebo-Controlled Trial
Cadegiani, Repurposing existing drugs for COVID-19: an endocrinology perspective, BMC Endocr Disord, doi:10.1186/s12902-020-00626-0
Flavio A Cadegiani, Fonseca, Mccoy, Zimerman, Nmirza et al., None
Goren, Wambier, Herrera, Mccoy, Vaño-Galván et al., Anti-androgens may protect against severe COVID-19 outcomes: results from a prospective cohort study of 77 hospitalized men, J Eur Acad Dermatol Venereol, doi:10.1111/jdv.16953
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell
Huffman, Rezq, Basnet, Cardozo, Romero, SARS-CoV-2 Viral Entry Proteins in Hyperandrogenemic Female Mice: Implications for Women with PCOS and COVID-19, Int J Mol Sci, doi:10.3390/ijms22094472
Li, Han, Dai, Distinct mechanisms for TMPRSS2 expression explain organ-specific inhibition of SARS-CoV-2 infection by enzalutamide, Nat Commun
Lin, Ferguson, White, Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2, Cancer Res
Mccoy, Cadegiani, Wambier, Herrera, Vaño-Galván et al., 5-alphareductase inhibitors are associated with reduced frequency of COVID-19 symptoms in males with androgenetic alopecia, J Eur Acad Dermatol Venereol, doi:10.1111/jdv.17021
Mccoy, Goren, Cadegiani, Vaño-Galván, Kovacevic et al., Proxalutamide (GT0918) Reduces the Rate of Hospitalization for COVID-19 Male Outpatients: A Randomized Double-Blinded Placebo-Controlled Trial, Front. Med, doi:10.3389/fmed.2021.668698
Min, Heberer, Gao, Becker, Loeb et al., A population-level analysis of the protective effects of androgen deprivation therapy against COVID-19 disease incidence and severity, medRxiv, doi:10.1101/2021.05.10
Qiao, Wang, Mannan, Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and TMPRSS2, Proc Natl Acad Sci U S A
Qu, Gu, Wang, Metabolomic profiling to evaluate the efficacy of proxalutamide, a novel androgen receptor antagonist, in prostate cancer cells, Invest New Drugs
Subramanian, Anand, Adderley, Okoth, Toulis et al., Increased COVID-19 infections in women with polycystic ovary syndrome: a population-based study, Eur J Endocrinol, doi:10.1530/EJE-20-1163
Wambier, Mehta, Goren, Cadegiani, COVID-19, androgens, and androgenetic alopecia, Derm Rev, doi:10.1002/der2.50
Wambier, Vaño-Galván, Mccoy, Gomez-Zubiaur, Herrera et al., Androgenetic alopecia present in the majority of patients hospitalized with COVID-19: The "Gabrin sign, J Am Acad Dermatol
Wambier, Vaño-Galván, Mccoy, Pai, Dhurat et al., Androgenetic alopecia in COVID-19: Compared to age-matched epidemiologic studies and hospital outcomes with or without the Gabrin sign, J Am Acad Dermatol, doi:10.1016/j.jaad.2020.07.099
Wu, Miao, Zhou, Suppression of Androgen Receptor (AR)-ACE2/TMPRSS2 Axis by AR Antagonists May Be Therapeutically Beneficial for Male COVID-19 Patients, SSRN Electron J
DOI record:
{
"DOI": "10.1101/2021.06.28.21259661",
"URL": "http://dx.doi.org/10.1101/2021.06.28.21259661",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Introduction</jats:title><jats:p>Proxalutamide, a second-generation non-steroidal antiandrogen (NSAA), primarily developed for castration-resistant prostate cancer, demonstrated reduction in 28-day mortality rate of 77.7% in hospitalized COVID-19 patients in a double-blind, placebo-controlled, two-arm randomized clinical trial (RCT), through intention-to-treat (ITT) analysis. We observed a high 28-day mortality rate of patients that did not complete the 14-day treatment with proxalutamide, compared to the placebo arm. These differences may raise hypotheses to explain the wide differences between ITT and on-treatment (OT) analysis in terms of efficacy. Despite the inherent limitations of OT analysis, we aimed to respond to unanswered questions regarding the drug efficacy when the 14-day treatment with proxalutamide was complete, and secondarily understand the causality relationship between treatment interruption and mortality rate.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>This is a <jats:italic>post-hoc</jats:italic> exploratory analysis of a double-blinded, randomized, placebo-controlled, prospective, multicentric, two-arm RCT of 300mg-daily 14-day proxalutamide therapy for hospitalized COVID-19 patients not requiring mechanical ventilation. OT population excluded patients that did not complete the full 14-day course of therapy or died from COVID-19 complications within 24 hours of randomization. The primary outcome was the 28-day COVID-19 mortality rate. Secondary outcomes included median hospital length, 14-day and 28-day alive hospital discharge rate and 28-day all-cause mortality rate of those who discontinued intervention.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>In total, 580 patients completed the 14-day treatment or died during treatment, including 288 patients in the proxalutamide arm and 292 patients in the placebo arm, with similar baseline characteristics between groups. The 28-day COVID-19 mortality rate was 4.2% in the proxalutamide group and 49.0% in the placebo group. The mortality risk ratio (RR) was 0.08 (95% CI, 0.05-0.15), with a number needed to treat (NNT) of 2.2 to prevent death. The median hospital length stay after randomization was 5 days (interquartile range [IQR] = 3 to 7.2 days) in the proxalutamide group and 9 days (IQR = 6 to 15 days) in the placebo group (p <0.001). The 28-day all-cause mortality rate of patients that received proxalutamide but interrupted treatment before 14 days was 79.3%, while those that received placebo and interrupted before 14 days was 52.8% (p = 0.054 between groups).</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>The reduction in 28-day all-cause mortality rate with 14-day proxalutamide treatment for hospitalized COVID-19 patients was more significant while on treatment adhesion (92%), compared to the reduction when all patients enrolled in the proxalutamide arm were considered (77.7%). However, the magnitude of statistical significance of the reduction in all-cause mortality and the NNT were similar between the OT and ITT analysis. The apparent high mortality risk rate with early interruption of proxalutamide treatments suggests that strategies for treatment compliance should be reinforced for future RCTs with proxalutamide. (<jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"clintrialgov\" xlink:href=\"NCT04728802\">NCT04728802</jats:ext-link>)</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2021,
7,
13
]
]
},
"author": [
{
"affiliation": [],
"family": "Zimerman",
"given": "Ricardo Ariel",
"sequence": "first"
},
{
"affiliation": [],
"family": "do Nascimento Fonseca",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "do Nascimento Correia",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barros",
"given": "Renan Nascimento",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Onety",
"given": "Dirce Costa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Israel",
"given": "Karla Cristina Petruccelli",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guerreiro",
"given": "Emilyn Oliveira",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Medeiros",
"given": "José Erique Miranda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nicolau",
"given": "Raquel Neves",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nicolau",
"given": "Luiza Fernanda Mendonça",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cunha",
"given": "Rafael Xavier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barroco",
"given": "Maria Fernanda Rodrigues",
"sequence": "additional"
},
{
"affiliation": [],
"family": "da Silva",
"given": "Patrícia Souza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Souza Paulain",
"given": "Raysa Wanzeller",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thompson",
"given": "Claudia Elizabeth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goren",
"given": "Andy",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4636-4489",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wambier",
"given": "Carlos Gustavo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2699-4344",
"affiliation": [],
"authenticated-orcid": false,
"family": "Cadegiani",
"given": "Flávio Adsuara",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
7,
2
]
],
"date-time": "2021-07-02T22:15:32Z",
"timestamp": 1625264132000
},
"deposited": {
"date-parts": [
[
2021,
7,
15
]
],
"date-time": "2021-07-15T12:55:56Z",
"timestamp": 1626353756000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2023,
3,
15
]
],
"date-time": "2023-03-15T14:31:50Z",
"timestamp": 1678890710498
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 4,
"issued": {
"date-parts": [
[
2021,
7,
2
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.06.28.21259661",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
7,
2
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
7,
2
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"key": "2021071505550685000_2021.06.28.21259661v2.1",
"unstructured": "WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus Disease (COVID-19) Dashboard."
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor",
"doi-asserted-by": "crossref",
"first-page": "271",
"issue": "2",
"journal-title": "Cell [Internet]",
"key": "2021071505550685000_2021.06.28.21259661v2.2",
"volume": "181",
"year": "2020"
},
{
"article-title": "Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2",
"first-page": "4180",
"issue": "17",
"journal-title": "Cancer Res [Internet]",
"key": "2021071505550685000_2021.06.28.21259661v2.3",
"volume": "59",
"year": "1999"
},
{
"DOI": "10.1016/j.jaad.2020.05.079",
"doi-asserted-by": "publisher",
"key": "2021071505550685000_2021.06.28.21259661v2.4"
},
{
"DOI": "10.1016/j.jaad.2020.07.099",
"doi-asserted-by": "publisher",
"key": "2021071505550685000_2021.06.28.21259661v2.5"
},
{
"DOI": "10.1136/bcr-2021-241572",
"doi-asserted-by": "publisher",
"key": "2021071505550685000_2021.06.28.21259661v2.6"
},
{
"DOI": "10.1111/jdv.17004",
"doi-asserted-by": "publisher",
"key": "2021071505550685000_2021.06.28.21259661v2.7"
},
{
"DOI": "10.3390/ijms22094472",
"doi-asserted-by": "publisher",
"key": "2021071505550685000_2021.06.28.21259661v2.8"
},
{
"DOI": "10.1530/EJE-20-1163",
"doi-asserted-by": "publisher",
"key": "2021071505550685000_2021.06.28.21259661v2.9"
},
{
"DOI": "10.1002/der2.50",
"doi-asserted-by": "crossref",
"key": "2021071505550685000_2021.06.28.21259661v2.10",
"unstructured": "Wambier CG , Mehta N , Goren A , Cadegiani FA . COVID-19, androgens, and androgenetic alopecia. Derm Rev. 2020;1–8. First published: 23 December 2020/ DOI: https://doi.org/10.1002/der2.50"
},
{
"DOI": "10.1186/s12902-020-00626-0",
"doi-asserted-by": "publisher",
"key": "2021071505550685000_2021.06.28.21259661v2.11"
},
{
"DOI": "10.2139/ssrn.3580526",
"doi-asserted-by": "crossref",
"key": "2021071505550685000_2021.06.28.21259661v2.12",
"unstructured": "Wu S , Miao L , Zhou Q , et al. Suppression of Androgen Receptor (AR)-ACE2/TMPRSS2 Axis by AR Antagonists May Be Therapeutically Beneficial for Male COVID-19 Patients. SSRN Electron J 2020;"
},
{
"DOI": "10.1073/pnas.2021450118",
"doi-asserted-by": "crossref",
"key": "2021071505550685000_2021.06.28.21259661v2.13",
"unstructured": "Qiao Y , Wang XM , Mannan R , et al. Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and TMPRSS2. Proc Natl Acad Sci U S A 2020;118(1)."
},
{
"DOI": "10.1038/s41467-021-21171-x",
"article-title": "Distinct mechanisms for TMPRSS2 expression explain organ-specific inhibition of SARS-CoV-2 infection by enzalutamide",
"doi-asserted-by": "crossref",
"first-page": "866",
"issue": "1",
"journal-title": "Nat Commun [Internet]",
"key": "2021071505550685000_2021.06.28.21259661v2.14",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1111/jdv.17021",
"doi-asserted-by": "publisher",
"key": "2021071505550685000_2021.06.28.21259661v2.15"
},
{
"DOI": "10.1111/jdv.16953",
"doi-asserted-by": "publisher",
"key": "2021071505550685000_2021.06.28.21259661v2.16"
},
{
"DOI": "10.1101/2021.05.10.21255146",
"doi-asserted-by": "crossref",
"key": "2021071505550685000_2021.06.28.21259661v2.17",
"unstructured": "Kyung Min Lee , Kent Heberer , Anthony Gao , Daniel J Becker , Stacy Loeb , Danil V. Makarov , Barbara Gulanski , Scott L DuVall , Mihaela Aslan , Jennifer Lee , Richard Hauger , Mei Chiung Shih , Julie Lynch , Matthew Rettig . A population-level analysis of the protective effects of androgen deprivation therapy against COVID-19 disease incidence and severity. medRxiv 2021.05.10.21255146; doi:https://doi.org/10.1101/2021.05.10.21255146"
},
{
"DOI": "10.7759/cureus.13047",
"doi-asserted-by": "publisher",
"key": "2021071505550685000_2021.06.28.21259661v2.18"
},
{
"DOI": "10.1007/s10637-020-00901-w",
"article-title": "Metabolomic profiling to evaluate the efficacy of proxalutamide, a novel androgen receptor antagonist, in prostate cancer cells",
"doi-asserted-by": "crossref",
"first-page": "1292",
"issue": "5",
"journal-title": "Invest New Drugs [Internet]",
"key": "2021071505550685000_2021.06.28.21259661v2.19",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.3389/fmed.2021.668698",
"doi-asserted-by": "publisher",
"key": "2021071505550685000_2021.06.28.21259661v2.20"
},
{
"DOI": "10.7759/cureus.13492",
"doi-asserted-by": "crossref",
"key": "2021071505550685000_2021.06.28.21259661v2.21",
"unstructured": "Cadegiani FA , McCoy J , Gustavo Wambier C , et al. Proxalutamide Significantly Accelerates Viral Clearance and Reduces Time to Clinical Remission in Patients with Mild to Moderate COVID-19: Results from a Randomized, Double-Blinded, Placebo-Controlled Trial. Cureus [Internet] 2021;Available from: https://www.cureus.com/articles/52299-proxalutamide-significantly-accelerates-viral-clearance-and-reduces-time-to-clinical-remission-in-patients-with-mild-to-moderate-covid-19-results-from-a-randomized-double-blinded-placebo-controlled-trial."
},
{
"DOI": "10.1101/2021.06.22.21259318",
"doi-asserted-by": "crossref",
"key": "2021071505550685000_2021.06.28.21259661v2.22",
"unstructured": "Flavio A Cadegiani , Daniel N Fonseca , John McCoy , Ricardo A. Zimerman , Fatima NMirza , Michael N Correia , Renan N Barros , Dirce C Onety , Karla Cristina P Israel , Brenda G Almeida , Emilyn O Guerreiro , Jose Enrique M Medeiros , Raquel N Nicolau , Luiza FM Nicolau , Rafael X Cunha , Maria Fernanda R Barroco , Patricia S da Silva , Gabriel S Ferreira , Flavio Renan PC Alcantara , Angelo M Ribeiro , Felipe O de Almeida , Adailson A de Souza , Suzyane S do Rosario , Raysa WS Paulain , Alessandra Reis MarissaLi , Claudia E Thompson , Gerald J Nau , Carlos Gustavo Wambier , Andy Goren . Efficacy of Proxalutamide in Hospitalized COVID-19 Patients: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Design Clinical Trial. medRxiv 2021.06.22.21259318; doi:https://doi.org/10.1101/2021.06.22.21259318."
},
{
"DOI": "10.1016/S2213-2600(20)30560-9",
"doi-asserted-by": "publisher",
"key": "2021071505550685000_2021.06.28.21259661v2.23"
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"article-title": "A minimal common outcome measure set for COVID-19 clinical research",
"doi-asserted-by": "crossref",
"first-page": "e192",
"issue": "8",
"journal-title": "Lancet Infect Dis [Internet]",
"key": "2021071505550685000_2021.06.28.21259661v2.24",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1002/sim.1296",
"doi-asserted-by": "publisher",
"key": "2021071505550685000_2021.06.28.21259661v2.25"
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2021.06.28.21259661"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "Proxalutamide Reduction of Mortality Rate in Hospitalized COVID-19 Patients Depends on Treatment Duration – an Exploratory Analysis of the Proxa-Rescue AndroCoV Trial",
"type": "posted-content"
}