Molnupiravir Use and 30-Day Hospitalizations or Death in Previously Uninfected Non-hospitalized High-risk Population with COVID-19
et al., The Journal of Infectious Diseases, doi:10.1093/infdis/jiad195, Jun 2023
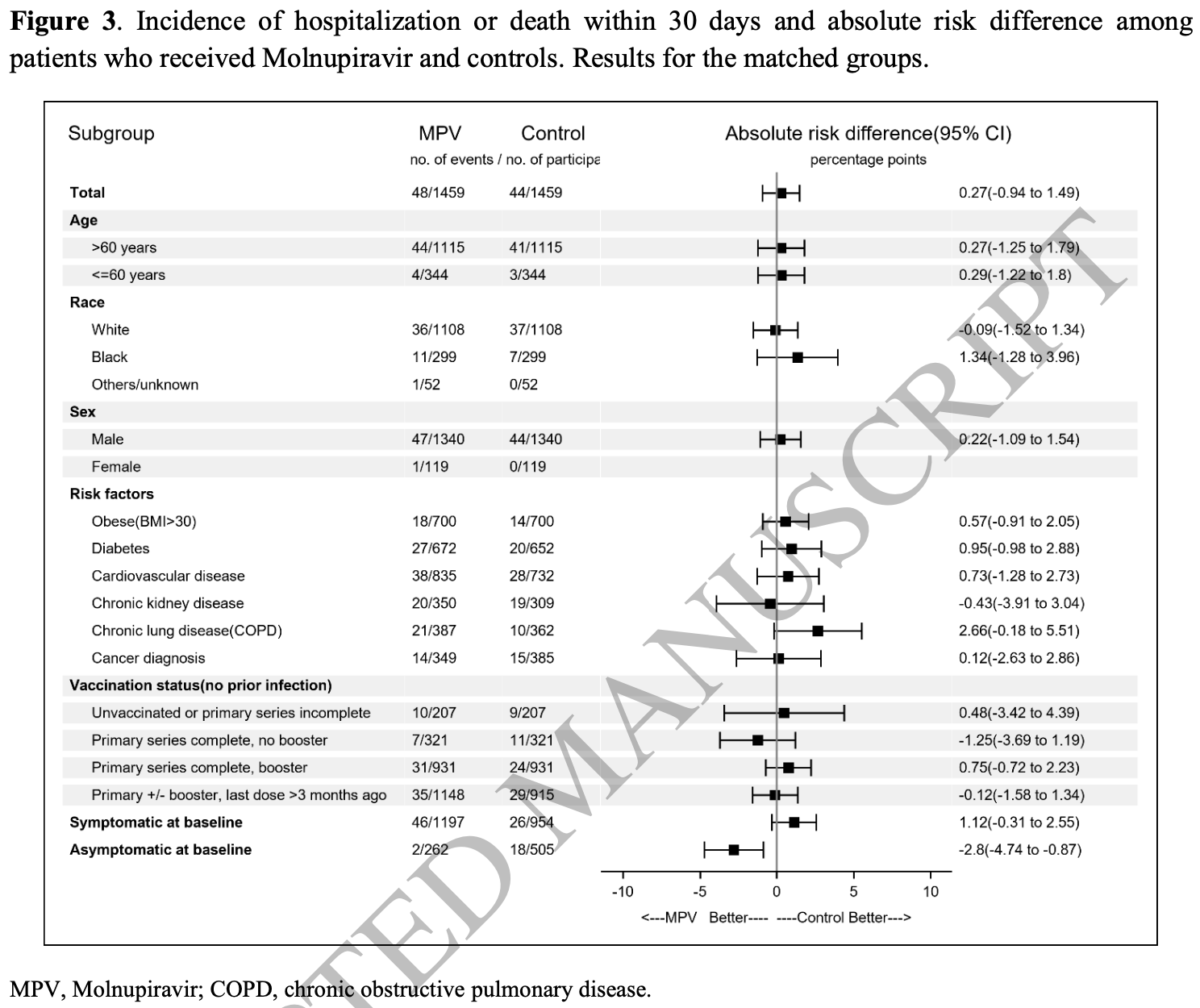
Retrospective 65,010 veterans in the USA, showing no significant difference in hospitalization/death with molnupiravir treatment. 1,729 patients received molnupiravir. Authors emulate a target trial closely matching the MOVe-OUT RCT and using 1,459 matched pairs.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments25.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death/hospitalization, 9.1% higher, RR 1.09, p = 0.75, treatment 48 of 1,459 (3.3%), control 44 of 1,459 (3.0%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
23.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Butt et al., 1 Jun 2023, retrospective, USA, peer-reviewed, 6 authors.
Molnupiravir Use and 30-Day Hospitalizations or Death in a Previously Uninfected Nonhospitalized High-risk Population With COVID-19
The Journal of Infectious Diseases, doi:10.1093/infdis/jiad195
Background: Clinical benefit of Molnupiravir (MPV) in COVID-19 infected sub-populations is unclear.
Methods: We used a matched cohort study design to determine the rate of hospitalization or death within 30 days of COVID-19 diagnosis among MPV treated and untreated controls.
Author Contributions
Study concept and study design:
Role of the Funding Source There was no funding source.
Data Access Drs. Butt and Mr. Yan had complete access to the data at all times and accept responsibility for the integrity of this article.
References
Abdelmalek, Mousa, Azithromycin Misuse During the COVID-19 Pandemic: A Cross-Sectional Study from Jordan, Infect Drug Resist
Abu-Raddad, Chemaitelly, Butt, National Study Group for C-V. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants, N Engl J Med
Alishaq, Nafady-Hego, Jeremijenko, Risk factors for breakthrough SARS-CoV-2 infection in vaccinated healthcare workers, PloS one
Anderson, Rouphael, Widge, Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults, N Engl J Med
Baden, Sahly, Essink, Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine, N Engl J Med
Beigel, Tomashek, Dodd, Remdesivir for the Treatment of Covid-19 -Final Report, N Engl J Med
Bernal, Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med
Butt, Khan, Yan, Shaikh, Omer et al., Rate and risk factors for breakthrough SARS-CoV-2 infection after vaccination, J Infect
Butt, Nafady-Hego, Chemaitelly, Outcomes Among Patients with Breakthrough SARS-CoV-2 Infection After Vaccination, Int J Infect Dis
Butt, Omer, Yan, Shaikh, Mayr, SARS-CoV-2 Vaccine Effectiveness in a High-Risk National Population in a Real-World Setting, Ann Intern Med
Butt, Talisa, Shaikh, Omer, Mayr, Relative Vaccine Effectiveness of a SARS-CoV-2 mRNA Vaccine Booster Dose Against the Omicron Variant, Clin Infect Dis
Butt, Yan, Shaikh, Mayr, Omer, Rate and Risk Factors for Severe/Critical Disease Among Fully Vaccinated Persons With Breakthrough Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in a High-Risk National Population, Clin Infect Dis
Butt, Yan, Shaikh, Mayr, Outcomes among patients with breakthrough SARS-CoV-2 infection after vaccination in a high-risk national population, EClinicalMedicine
Chemaitelly, Tang, Hasan, Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar, N Engl J Med
Coronavirus, COVID-19) Update: FDA Authorizes First Oral Antiviral for Treatment of COVID-19
Coronavirus, Update: FDA Authorizes Additional Oral Antiviral for Treatment of COVID-19 in Certain Adults
Dagan, Barda, Kepten, BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting, N Engl J Med
Dougan, Nirula, Azizad, Bamlanivimab plus Etesevimab in Mild or Moderate Covid-19, N Engl J Med
Fernandez-Ruiz, Lopez-Medrano, Asin, Tocilizumab for the treatment of adult patients with severe COVID-19 pneumonia: A single-center cohort study, J Med Virol
Furlan, Caramelli, The regrettable story of the "Covid Kit" and the "Early Treatment of Covid-19, Lancet Reg Health Am
Goldman, Lye, Hui, Remdesivir for 5 or 10 Days in Patients with Severe Covid-19, N Engl J Med
Gottlieb, Nirula, Chen, Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial, JAMA
Group, Horby, Lim, Dexamethasone in Hospitalized Patients with Covid-19, N Engl J Med
Hacisuleyman, Hale, Saito, Vaccine Breakthrough Infections with SARS-CoV-2 Variants, N Engl J Med
Ioannou, Locke, Hare, COVID-19 Vaccination Effectiveness Against Infection or Death in a National U.S. Health Care System : A Target Trial Emulation Study, Ann Intern Med
Johnson, Puenpatom, Moncada, Effect of Molnupiravir on Biomarkers, Respiratory Interventions, and Medical Services in COVID-19 : A Randomized, Placebo-Controlled Trial, Ann Intern Med
Khoo, Fitzgerald, Saunders, Molnupiravir versus placebo in unvaccinated and vaccinated patients with early SARS-CoV-2 infection in the UK (AGILE CST-2): a randomised, placebo-controlled, double-blind, phase 2 trial, Lancet Infect Dis
Lee, Sun, Jang, Connelly, Misinformation of COVID-19 vaccines and vaccine hesitancy, Sci Rep
Lundgren, Grund, Barkauskas, A Neutralizing Monoclonal Antibody for Hospitalized Patients with Covid-19, N Engl J Med
Mayr, Talisa, Castro, Shaikh, Omer et al., COVID-19 disease severity in US Veterans infected during Omicron and Delta variant predominant periods, Nat Commun
Mayr, Talisa, Shaikh, Yende, Butt, Effectiveness of Homologous or Heterologous Covid-19 Boosters in Veterans, N Engl J Med, doi:10.1056/NEJMc2200415
Najjar-Debbiny, Gronich, Weber, Effectiveness of Molnupiravir in High Risk Patients: a Propensity Score Matched Analysis, Clin Infect Dis
Polack, Thomas, Kitchin, Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine, N Engl J Med
Price, Altice, Shyr, Tocilizumab Treatment for Cytokine Release Syndrome in Hospitalized Patients With Coronavirus Disease 2019: Survival and Clinical Outcomes, Chest
Romer, Winneg, Jamieson, Brensinger, Jamieson, Misinformation about vaccine safety and uptake of COVID-19 vaccines among adults and 5-11-year-olds in the United States, Vaccine
Shen, Wang, Zhao, Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma, JAMA
Somers, Eschenauer, Troost, Tocilizumab for Treatment of Mechanically Ventilated Patients With COVID-19, Clin Infect Dis
Voysey, Clemens, Madhi, Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK, Lancet
Wai, Chan, Cheung, Association of Molnupiravir and Nirmatrelvir-Ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19, Lancet Reg Health West Pac
Walsh, Frenck, Falsey, Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates, N Engl J Med
Weinreich, Sivapalasingam, Norton, REGEN-COV Antibody Combination and Outcomes in Outpatients with Covid-19, N Engl J Med
West, Charlton, Vaughan-Sarrazin, Dual use of VA and non-VA hospitals by Veterans with multiple hospitalizations, BMC Health Serv Res
DOI record:
{
"DOI": "10.1093/infdis/jiad195",
"ISSN": [
"0022-1899",
"1537-6613"
],
"URL": "http://dx.doi.org/10.1093/infdis/jiad195",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Clinical benefit of molnupiravir (MPV) in coronavirus disease 2019 (COVID-19)–infected subpopulations is unclear.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>We used a matched cohort study design to determine the rate of hospitalization or death within 30 days of COVID-19 diagnosis among MPV treated and untreated controls. Participants were nonhospitalized, previously uninfected Veterans with a first confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection between 1 January and 31 August 2022, who were prescribed MPV within 3 days of COVID-19 diagnosis, and matched individuals who were not prescribed MPV.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Among 1459 matched pairs, the incidence of hospitalization/death was not different among MPV treated versus untreated controls (48 vs 44 cases; absolute risk difference [ARD], 0.27; 95% confidence interval [CI], −.94 to 1.49). No benefit was observed among those &gt;60 or ≤60 years old (ARD, 0.27; 95% CI, −1.25 to 1.79 vs ARD, −0.29; 95% CI, −1.22 to 1.80), those with specific comorbidities, or by vaccination status. A significant benefit was observed in asymptomatic but not in symptomatic persons (ARD, −2.80; 95% CI, −4.74 to −.87 vs ARD, 1.12; 95% CI −.31 to 2.55). Kaplan-Meier curves did not show a difference in proportion of persons who were hospitalized or died among MPV treated compared with untreated controls (logrank P = .7).</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>MPV was not associated with a reduction in hospitalization or death within 30 days of COVID-19 diagnosis. A subgroup of patients presenting without symptoms experienced a benefit.</jats:p>\n </jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-1118-1826",
"affiliation": [
{
"name": "Veterans Affairs Pittsburgh Healthcare System , Pittsburgh, Pennsylvania , USA"
},
{
"name": "Department of Medicine, Weill Cornell Medicine , New York, New York , USA"
},
{
"name": "Department of Medicine, Weill Cornell Medicine , Doha , Qatar"
},
{
"name": "Department of Population Health Sciences, Weill Cornell Medicine , New York, New York , USA"
},
{
"name": "Department of Population Health Sciences, Weill Cornell Medicine , Doha , Qatar"
},
{
"name": "Hamad Medical Corporation , Doha , Qatar"
}
],
"authenticated-orcid": false,
"family": "Butt",
"given": "Adeel A",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Veterans Affairs Pittsburgh Healthcare System , Pittsburgh, Pennsylvania , USA"
}
],
"family": "Yan",
"given": "Peng",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Veterans Affairs Pittsburgh Healthcare System , Pittsburgh, Pennsylvania , USA"
},
{
"name": "Department of Medicine, Division of Gastroenterology, University of Pittsburgh , Pittsburgh, Pennsylvania , USA"
}
],
"family": "Shaikh",
"given": "Obaid S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute for Global Health, Yale University , New Haven, Connecticut , USA"
}
],
"family": "Omer",
"given": "Saad B",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Research, Investigation, and Systems Modeling of Acute Illness Center, Department of Critical Care Medicine, University of Pittsburgh , Pittsburgh, Pennsylvania , USA"
},
{
"name": "Department of Critical Care Medicine, University of Pittsburgh School of Medicine , Pittsburgh, Pennsylvania , USA"
}
],
"family": "Mayr",
"given": "Florian B",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Research, Investigation, and Systems Modeling of Acute Illness Center, Department of Critical Care Medicine, University of Pittsburgh , Pittsburgh, Pennsylvania , USA"
},
{
"name": "Department of Critical Care Medicine, University of Pittsburgh School of Medicine , Pittsburgh, Pennsylvania , USA"
}
],
"family": "Talisa",
"given": "Victor B",
"sequence": "additional"
}
],
"container-title": "The Journal of Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T13:34:19Z",
"timestamp": 1685626459000
},
"deposited": {
"date-parts": [
[
2023,
10,
18
]
],
"date-time": "2023-10-18T09:37:52Z",
"timestamp": 1697621872000
},
"funder": [
{
"DOI": "10.13039/100000002",
"award": [
"K23GM132688"
],
"doi-asserted-by": "publisher",
"name": "National Institutes of Health"
}
],
"indexed": {
"date-parts": [
[
2024,
2,
16
]
],
"date-time": "2024-02-16T10:19:04Z",
"timestamp": 1708078744429
},
"is-referenced-by-count": 3,
"issue": "8",
"issued": {
"date-parts": [
[
2023,
6,
1
]
]
},
"journal-issue": {
"issue": "8",
"published-online": {
"date-parts": [
[
2023,
6,
1
]
]
},
"published-print": {
"date-parts": [
[
2023,
10,
18
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://academic.oup.com/pages/standard-publication-reuse-rights",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
1
]
],
"date-time": "2023-06-01T00:00:00Z",
"timestamp": 1685577600000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/jid/advance-article-pdf/doi/10.1093/infdis/jiad195/50600051/jiad195.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/jid/article-pdf/228/8/1033/52210066/jiad195.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/jid/article-pdf/228/8/1033/52210066/jiad195.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"page": "1033-1041",
"prefix": "10.1093",
"published": {
"date-parts": [
[
2023,
6,
1
]
]
},
"published-online": {
"date-parts": [
[
2023,
6,
1
]
]
},
"published-other": {
"date-parts": [
[
2023,
10,
15
]
]
},
"published-print": {
"date-parts": [
[
2023,
10,
18
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"DOI": "10.1056/NEJMc2104974",
"article-title": "Effectiveness of the BNT162b2 COVID-19 vaccine against the B.1.1.7 and B.1.351 variants",
"author": "Abu-Raddad",
"doi-asserted-by": "crossref",
"first-page": "187",
"journal-title": "N Engl J Med",
"key": "2023101809355295300_jiad195-B1",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2035389",
"article-title": "Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine",
"author": "Baden",
"doi-asserted-by": "crossref",
"first-page": "403",
"journal-title": "N Engl J Med",
"key": "2023101809355295300_jiad195-B2",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2034577",
"article-title": "Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine",
"author": "Polack",
"doi-asserted-by": "crossref",
"first-page": "2603",
"journal-title": "N Engl J Med",
"key": "2023101809355295300_jiad195-B3",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2027906",
"article-title": "Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates",
"author": "Walsh",
"doi-asserted-by": "crossref",
"first-page": "2439",
"journal-title": "N Engl J Med",
"key": "2023101809355295300_jiad195-B4",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2028436",
"article-title": "Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults",
"author": "Anderson",
"doi-asserted-by": "crossref",
"first-page": "2427",
"journal-title": "N Engl J Med",
"key": "2023101809355295300_jiad195-B5",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2101765",
"article-title": "BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting",
"author": "Dagan",
"doi-asserted-by": "crossref",
"first-page": "1412",
"journal-title": "N Engl J Med",
"key": "2023101809355295300_jiad195-B6",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(20)32661-1",
"article-title": "Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK",
"author": "Voysey",
"doi-asserted-by": "crossref",
"first-page": "99",
"journal-title": "Lancet",
"key": "2023101809355295300_jiad195-B7",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.7326/M21-1577",
"article-title": "SARS-CoV-2 vaccine effectiveness in a high-risk national population in a real-world setting",
"author": "Butt",
"doi-asserted-by": "crossref",
"first-page": "1404",
"journal-title": "Ann Intern Med",
"key": "2023101809355295300_jiad195-B8",
"volume": "174",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2114114",
"article-title": "Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar",
"author": "Chemaitelly",
"doi-asserted-by": "crossref",
"first-page": "e83",
"journal-title": "N Engl J Med",
"key": "2023101809355295300_jiad195-B9",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1016/j.jinf.2021.05.021",
"article-title": "Rate and risk factors for breakthrough SARS-CoV-2 infection after vaccination",
"author": "Butt",
"doi-asserted-by": "crossref",
"first-page": "237",
"journal-title": "J Infect",
"key": "2023101809355295300_jiad195-B10",
"volume": "83",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2021.101117",
"article-title": "Outcomes among patients with breakthrough SARS-CoV-2 infection after vaccination in a high-risk national population",
"author": "Butt",
"doi-asserted-by": "crossref",
"first-page": "101117",
"journal-title": "EClinicalMedicine",
"key": "2023101809355295300_jiad195-B11",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2021.08.008",
"article-title": "Outcomes among patients with breakthrough SARS-CoV-2 infection after vaccination",
"author": "Butt",
"doi-asserted-by": "crossref",
"first-page": "353",
"journal-title": "Int J Infect Dis",
"key": "2023101809355295300_jiad195-B12",
"volume": "110",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0258820",
"article-title": "Risk factors for breakthrough SARS-CoV-2 infection in vaccinated healthcare workers",
"author": "Alishaq",
"doi-asserted-by": "crossref",
"first-page": "e0258820",
"journal-title": "PLoS One",
"key": "2023101809355295300_jiad195-B13",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2105000",
"article-title": "Vaccine breakthrough infections with SARS-CoV-2 variants",
"author": "Hacisuleyman",
"doi-asserted-by": "crossref",
"first-page": "2212",
"journal-title": "N Engl J Med",
"key": "2023101809355295300_jiad195-B14",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.4783",
"article-title": "Treatment of 5 critically ill patients with COVID-19 with convalescent plasma",
"author": "Shen",
"doi-asserted-by": "crossref",
"first-page": "1582",
"journal-title": "JAMA",
"key": "2023101809355295300_jiad195-B15",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1002/jmv.26308",
"article-title": "Tocilizumab for the treatment of adult patients with severe COVID-19 pneumonia: a single-center cohort study",
"author": "Fernandez-Ruiz",
"doi-asserted-by": "crossref",
"first-page": "831",
"journal-title": "J Med Virol",
"key": "2023101809355295300_jiad195-B16",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa954",
"article-title": "Tocilizumab for treatment of mechanically ventilated patients with COVID-19",
"author": "Somers",
"doi-asserted-by": "crossref",
"first-page": "e445",
"journal-title": "Clin Infect Dis",
"key": "2023101809355295300_jiad195-B17",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1016/j.chest.2020.06.006",
"article-title": "Tocilizumab treatment for cytokine release syndrome in hospitalized patients with coronavirus disease 2019: survival and clinical outcomes",
"author": "Price",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "Chest",
"key": "2023101809355295300_jiad195-B18",
"volume": "158",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of COVID-19—final report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "2023101809355295300_jiad195-B19",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2015301",
"article-title": "Remdesivir for 5 or 10 days in patients with severe COVID-19",
"author": "Goldman",
"doi-asserted-by": "crossref",
"first-page": "1827",
"journal-title": "N Engl J Med",
"key": "2023101809355295300_jiad195-B20",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2102685",
"article-title": "Bamlanivimab plus etesevimab in mild or moderate COVID-19",
"author": "Dougan",
"doi-asserted-by": "crossref",
"first-page": "1382",
"journal-title": "N Engl J Med",
"key": "2023101809355295300_jiad195-B21",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2033130",
"article-title": "A neutralizing monoclonal antibody for hospitalized patients with COVID-19",
"author": "Lundgren",
"doi-asserted-by": "crossref",
"first-page": "905",
"journal-title": "N Engl J Med",
"key": "2023101809355295300_jiad195-B22",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.0202",
"article-title": "Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "632",
"journal-title": "JAMA",
"key": "2023101809355295300_jiad195-B23",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2108163",
"article-title": "REGEN-COV antibody combination and outcomes in outpatients with COVID-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "e81",
"journal-title": "N Engl J Med",
"key": "2023101809355295300_jiad195-B24",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with COVID-19",
"author": "Group",
"doi-asserted-by": "crossref",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "2023101809355295300_jiad195-B25",
"volume": "384",
"year": "2021"
},
{
"author": "Food and Drug Administration",
"key": "2023101809355295300_jiad195-B26",
"year": "2021"
},
{
"author": "Food and Drug Administration",
"key": "2023101809355295300_jiad195-B27",
"year": "2021"
},
{
"author": "Food and Drug Administration",
"key": "2023101809355295300_jiad195-B28",
"year": "2021"
},
{
"author": "Food and Drug Administration",
"key": "2023101809355295300_jiad195-B29",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "2023101809355295300_jiad195-B30",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.7326/M22-0729",
"article-title": "Effect of molnupiravir on biomarkers, respiratory interventions, and medical services in COVID-19 : a randomized, placebo-controlled trial",
"author": "Johnson",
"doi-asserted-by": "crossref",
"first-page": "1126",
"journal-title": "Ann Intern Med",
"key": "2023101809355295300_jiad195-B31",
"volume": "175",
"year": "2022"
},
{
"DOI": "10.1016/j.lanwpc.2022.100602",
"article-title": "Association of molnupiravir and nirmatrelvir-ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19",
"author": "Wai",
"doi-asserted-by": "crossref",
"first-page": "100602",
"journal-title": "Lancet Reg Health West Pac",
"key": "2023101809355295300_jiad195-B32",
"volume": "30",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(22)00644-2",
"article-title": "Molnupiravir versus placebo in unvaccinated and vaccinated patients with early SARS-CoV-2 infection in the UK (AGILE CST-2): a randomised, placebo-controlled, double-blind, phase 2 trial",
"author": "Khoo",
"doi-asserted-by": "crossref",
"first-page": "183",
"journal-title": "Lancet Infect Dis",
"key": "2023101809355295300_jiad195-B33",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac781",
"article-title": "Effectiveness of molnupiravir in high risk patients: a propensity score matched analysis",
"author": "Najjar-Debbiny",
"doi-asserted-by": "crossref",
"first-page": "453",
"journal-title": "Clin Infect Dis",
"key": "2023101809355295300_jiad195-B34",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciab1023",
"article-title": "Rate and risk factors for severe/critical disease among fully vaccinated persons with breakthrough severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in a high-risk national population",
"author": "Butt",
"doi-asserted-by": "crossref",
"first-page": "e849",
"journal-title": "Clin Infect Dis",
"key": "2023101809355295300_jiad195-B35",
"volume": "75",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2200415",
"article-title": "Effectiveness of homologous or heterologous COVID-19 boosters in veterans",
"author": "Mayr",
"doi-asserted-by": "crossref",
"first-page": "1375",
"journal-title": "N Engl J Med",
"key": "2023101809355295300_jiad195-B36",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1038/s41467-022-31402-4",
"article-title": "COVID-19 disease severity in US veterans infected during omicron and Delta variant predominant periods",
"author": "Mayr",
"doi-asserted-by": "crossref",
"first-page": "3647",
"journal-title": "Nat Commun",
"key": "2023101809355295300_jiad195-B37",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.7326/M21-3256",
"article-title": "COVID-19 vaccination effectiveness against infection or death in a national U.S. health care system: a target trial emulation study",
"author": "Ioannou",
"doi-asserted-by": "crossref",
"first-page": "352",
"journal-title": "Ann Intern Med",
"key": "2023101809355295300_jiad195-B38",
"volume": "175",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac328",
"article-title": "Relative vaccine effectiveness of a SARS-CoV-2 mRNA vaccine booster dose against the omicron variant",
"author": "Butt",
"doi-asserted-by": "crossref",
"first-page": "2161",
"journal-title": "Clin Infect Dis",
"key": "2023101809355295300_jiad195-B39",
"volume": "75",
"year": "2022"
},
{
"author": "SAS Institute",
"key": "2023101809355295300_jiad195-B40"
},
{
"article-title": "The regrettable story of the “COVID kit” and the “early treatment of COVID-19” in Brazil",
"author": "Furlan",
"first-page": "100089",
"journal-title": "Lancet Reg Health Am",
"key": "2023101809355295300_jiad195-B41",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.2147/IDR.S351827",
"article-title": "Azithromycin misuse during the COVID-19 pandemic: a cross-sectional study from Jordan",
"author": "Abdelmalek",
"doi-asserted-by": "crossref",
"first-page": "747",
"journal-title": "Infect Drug Resist",
"key": "2023101809355295300_jiad195-B42",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1016/j.vaccine.2022.09.046",
"article-title": "Misinformation about vaccine safety and uptake of COVID-19 vaccines among adults and 5–11-year-olds in the United States",
"author": "Romer",
"doi-asserted-by": "crossref",
"first-page": "6463",
"journal-title": "Vaccine",
"key": "2023101809355295300_jiad195-B43",
"volume": "40",
"year": "2022"
},
{
"DOI": "10.1038/s41598-022-17430-6",
"article-title": "Misinformation of COVID-19 vaccines and vaccine hesitancy",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "13681",
"journal-title": "Sci Rep",
"key": "2023101809355295300_jiad195-B44",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1186/s12913-015-1069-8",
"article-title": "Dual use of VA and non-VA hospitals by veterans with multiple hospitalizations",
"author": "West",
"doi-asserted-by": "crossref",
"first-page": "431",
"journal-title": "BMC Health Serv Res",
"key": "2023101809355295300_jiad195-B45",
"volume": "15",
"year": "2015"
}
],
"reference-count": 45,
"references-count": 45,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/jid/article/228/8/1033/7187858"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Immunology and Allergy"
],
"subtitle": [],
"title": "Molnupiravir Use and 30-Day Hospitalizations or Death in a Previously Uninfected Nonhospitalized High-risk Population With COVID-19",
"type": "journal-article",
"volume": "228"
}