ALBACOVIDIOL Study: Effect of Calcifediol Treatment on Mortality in Patients Hospitalized for COVID-19: A Retrospective Analysis
et al., Nutrients, doi:10.3390/nu17121968, ALBACOVIDIOL, NCT05819918, Jun 2025
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 135 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 230 hospitalized COVID-19 patients in Spain, showing lower mortality with calcifediol treatment (p = 0.053).
Meta-analysis shows that late stage treatment with calcitriol / calcifediol (or
paricalcitol, alfacalcidol, etc.) is more effective than cholecalciferol: 66% [47‑78%] lower risk vs. 45% [34‑54%] lower risk.
Cholecalciferol requires two hydroxylation steps to become activated - first
in the liver to calcifediol, then in the kidney to calcitriol. Calcitriol,
paricalcitol, and alfacalcidol are active vitamin D analogs that do not
require conversion. This allows them to have more rapid onset of action
compared to cholecalciferol. The time delay for cholecalciferol to increase
serum calcifediol levels can be 2-3 days, and the delay for converting
calcifediol to active calcitriol can be up to 7 days.
This is the 133rd of 135 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
40 studies are RCTs, which show efficacy with p=0.0000001.
|
risk of death, 52.4% lower, OR 0.48, p = 0.05, treatment 119, control 111, adjusted per study, all patients, multivariable, RR approximated with OR.
|
|
risk of death, 95.4% lower, OR 0.05, p = 0.004, treatment 16, control 18, severely deficient patients, RR approximated with OR.
|
|
risk of death, 38.5% lower, RR 0.62, p = 0.19, treatment 8 of 65 (12.3%), control 33 of 165 (20.0%), NNT 13, previous vitamin D treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Blázquez-Cabrera et al., 10 Jun 2025, retrospective, Spain, peer-reviewed, mean age 73.0, 6 authors, study period 24 January, 2021 - 8 March, 2021, dosage varies (calcitriol), trial NCT05819918 (history) (ALBACOVIDIOL).
Contact: roger.bouillon@kuleuven.be.
ALBACOVIDIOL Study: Effect of Calcifediol Treatment on Mortality in Patients Hospitalized for COVID-19: A Retrospective Analysis
Nutrients, doi:10.3390/nu17121968
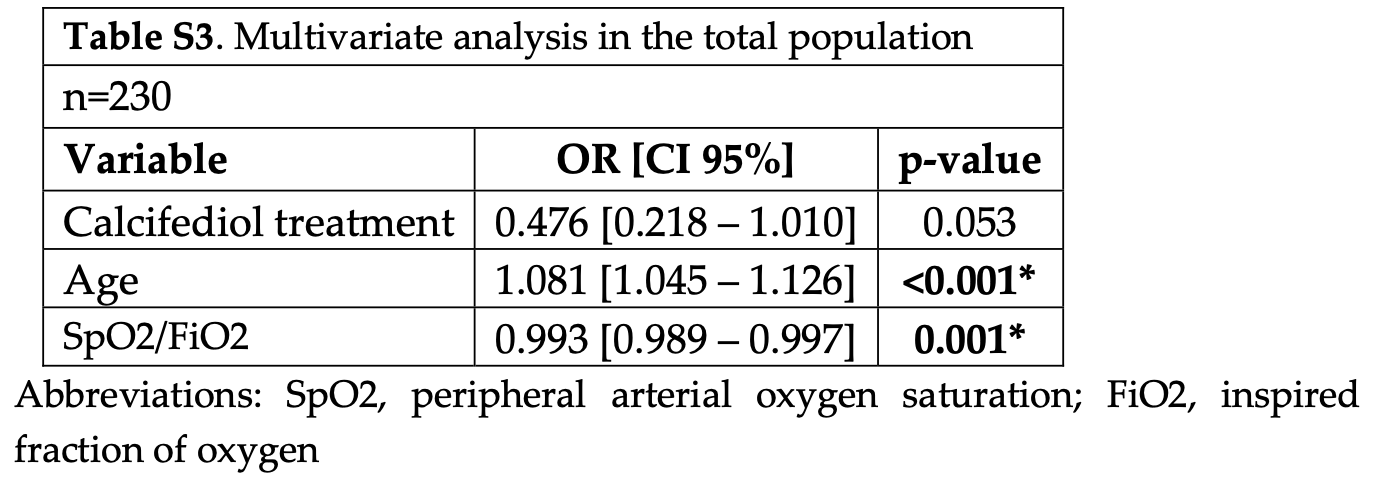
Background: Immunomodulatory treatments targeting excessive host immune responses favorably shifting the course of COVID-19. High doses of calcifediol may reduce the mortality of this infection. Objective: To evaluate how a high dose of calcifediol modifies the risk of death in patients hospitalized with COVID-19 during the first outbreaks. Design: A retrospective, observational study to evaluate the relationship between treatment with calcifediol and the risk of death in patients hospitalized with COVID-19 at the "Complejo Hospitalario Universitario de Albacete" (CHUA), Spain, during the months of January to March 2021. Patients were treated with corticosteroids, and some patients also received baricitinib and/or high doses of calcifediol, according to CHUA's therapeutic protocol 2021 for COVID-19. The primary outcome measure was mortality according to calcifediol treatment. Results: A total of 230 patients were included. 25(OH)D levels were measured on admission in 148 patients, showing a high prevalence of vitamin D deficiency [median 25(OH)D: 17.5 ng/mL]. Thirty-four (23%) had severe deficiency (25(OH)D ≤ 10 ng/mL). In the 119 patients (51.7%) who received in-hospital treatment with a high dose of calcifediol, the mortality rate was 12.6% (15 cases, 95% confidence interval [CI], 7.8-19.8%), while in 111 patients who did not receive treatment with calcifediol, the death rate was 23.4% (26 cases, 95% CI: 16.5-32.1%; p = 0.039). The odds ratio (OR) in treated vs. untreated patients was 0.47 (95% CI: 0.23-0.95). Among the patients admitted with severe deficiency, 16 received treatment with calcifediol, with a mortality rate of 0.0% (0 cases, 95% CI: 0.0-19.4%), while in the 18 not treated with calcifediol, a death rate of 38.9% was observed (7 cases, 95% CI: 20.3-61.4%; p = 0.008). The mortality rate was lower in patients treated with the combination of calcifediol and corticosteroids vs. those treated with corticosteroids alone (p = 0.038) and vs. those treated with corticosteroids and baricitinib (p = 0.033). Conclusions: In the ALBACOVIDIOL study, calcifediol treatment was associated with a lower observed mortality rate in hospitalized patients with COVID-19 treated with corticosteroids (with or without baricitinib), especially in those with severe vitamin D deficiency. Causality cannot be inferred due to the retrospective study design.
Informed Consent Statement: The Biomedical Research Ethics Committee of the Complejo Hospitalario Universitario de Albacete (CHUA), Spain approved the study for secondary use of clinical data for research purposes. The study did not require informed consent as the investigators handled completely anonymized data without any reference to personal data that could allow identification by any means. All research was conducted in accordance with the relevant guidelines and regulations.
Conflicts of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Appendix A
Collaborators in Data Collection Teresa Granero-Salas, Andrea Pérez-Trujillo, Marta Guzman-Pérez, Marcos-Alexander Ostaiza-Ordoñez, Rocio Garvi-Merino, Jordi Olucha-Puchol, Cristina Garcia-Gomez, Cristina del Pozo-Carlavilla, Belen Serna-Serrano, Juan Manuel Collado-Sanz, Hector Alabort-Ayllón, Beira da Silva-Cabañero.
References
Abani, Abbas, Abbas, Abbas, Abbas et al., Baricitinib in Patients Admitted to Hospital with COVID-19 (RECOVERY): A Randomised, Controlled, Open-Label, Platform Trial and Updated Meta-Analysis, Lancet, doi:10.1016/S0140-6736(22)01109-6
Alcala-Diaz, Limia-Perez, Gomez-Huelgas, Martin-Escalante, Cortes-Rodriguez et al., Calcifediol Treatment and Hospital Mortality Due to Covid-19: A Cohort Study, Nutrients, doi:10.3390/nu13061760
Altmann, Whettlock, Liu, Arachchillage, Boyton, The Immunology of Long COVID, Nat. Rev. Immunol, doi:10.1038/s41577-023-00904-7
Bastard, Rosen, Zhang, Michailidis, Hoffmann et al., Autoantibodies against Type I IFNs in Patients with Life-Threatening COVID-19, Science, doi:10.1126/science.abd4585
Bischoff-Ferrari, Dawson-Hughes, Stöcklin, Sidelnikov, Willett et al., Oral Supplementation with 25(OH)D 3 versus Vitamin D 3 : Effects on 25(OH)D Levels, Lower Extremity Function, Blood Pressure, and Markers of Innate Immunity, J. Bone Miner. Res, doi:10.1002/jbmr.551
Bishop, Ismailova, Dimeloe, Hewison, White, Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory, JBMR Plus, doi:10.1002/jbm4.10405
Blanco-Melo, Nilsson-Payant, Liu, Uhl, Hoagland et al., Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19, Cell, doi:10.1016/j.cell.2020.04.026
Bouillon, Bikle, Vitamin D Metabolism Revised: Fall of Dogmas, J. Bone Miner. Res, doi:10.1002/jbmr.3884
Bouillon, Quesada-Gomez, Vitamin D Endocrine System and COVID-19, JBMR Plus, doi:10.1002/jbm4.10576
Caiazzo, Rezig, Bruzzese, Ialenti, Cicala et al., Systemic Administration of Glucocorticoids, Cardiovascular Complications and Mortality in Patients Hospitalised with COVID-19, SARS, MERS or Influenza: A Systematic Review and Meta-Analysis of Randomised Trials, Pharmacol. Res, doi:10.1016/j.phrs.2021.106053
Cain, Cidlowski, Immune Regulation by Glucocorticoids, Nat. Rev. Immunol, doi:10.1038/nri.2017.1
Capano, Howlett, Jarvis, Ramesh, Long-Term Policy Impacts of the Coronavirus: Normalization, Adaptation, and Acceleration in the Post-COVID State, Policy Soc, doi:10.1093/polsoc/puab018
Castillo, Entrenas Costa, Vaquero Barrios, Alcalá Díaz, López Miranda et al., Effect of Calcifediol Treatment and Best Available Therapy versus Best Available Therapy on Intensive Care Unit Admission and Mortality among Patients Hospitalized for COVID-19: A Pilot Randomized Clinical Study, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2020.105751
Charlson, Pompei, Ales, Mackenzie, A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation, J. Chronic Dis, doi:10.1016/0021-9681(87)90171-8
Chauss, Freiwald, Mcgregor, Yan, Wang et al., Autocrine Vitamin D Signaling Switches off Pro-Inflammatory Programs of TH1 Cells, Nat. Immunol, doi:10.1038/s41590-021-01080-3
Dagens, Sigfrid, Cai, Lipworth, Cheung et al., Quality, and Inclusivity of Clinical Guidelines Produced Early in the Covid-19 Pandemic: Rapid Review, BMJ, doi:10.1136/bmj.m1936
Damsky, Peterson, Ramseier, Al-Bawardy, Chun et al., The Emerging Role of Janus Kinase Inhibitors in the Treatment of Autoimmune and Inflammatory Diseases, J. Allergy Clin. Immunol, doi:10.1016/j.jaci.2020.10.022
Deeks, Higgins, Statistical Algorithms in Review Manager 5
Diaz-Curiel, Cabello, Arboiro-Pinel, Mansur, Heili-Frades et al., The Relationship between 25(OH) Vitamin D Levels and COVID-19 Onset and Disease Course in Spanish Patients, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2021.105928
Efird, Anderson, Jindal, Suzuki, Interaction of Vitamin D and Corticosteroid Use in Hospitalized COVID-19 Patients: A Potential Explanation for Inconsistent Findings in the Literature, Curr. Pharm. Des, doi:10.2174/1381612828666220418132847
Entrenas-Castillo, Entrenas-Costa, Pata, Gámez, Muñoz-Corroto et al., Latent Class Analysis Reveals, in Patient Profiles, COVID-19-Related Better Prognosis by Calcifediol Treatment than Glucocorticoids, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2024.106609
Entrenas-Castillo, Entrenas-Costa, Pata, Jurado-Gamez, Muñoz-Corroto et al., Calcifediol or Corticosteroids in the Treatment of COVID-19: An Observational Study, Nutrients, doi:10.3390/nu16121910
González-Molero, Morcillo, Valdés, Pérez-Valero, Botas et al., Vitamin D Deficiency in Spain: A Population-Based Cohort Study, Eur. J. Clin. Nutr, doi:10.1038/ejcn.2010.265
Griffith, Morris, Tudball, Herbert, Mancano et al., Collider Bias Undermines Our Understanding of COVID-19 Disease Risk and Severity, Nat. Commun, doi:10.1038/s41467-020-19478-2
Group, Dexamethasone in Hospitalized Patients with Covid-19, N. Engl. J. Med, doi:10.1056/nejmoa2021436
Guan, Ni, Hu, Liang, Ou et al., Clinical Characteristics of Coronavirus Disease 2019 in China, N. Engl. J. Med, doi:10.1056/NEJMoa2002032
Gupta, Madhavan, Sehgal, Nair, Mahajan et al., Extrapulmonary Manifestations of COVID-19, Nat. Med, doi:10.1038/s41591-020-0968-3
Hafezi, Saheb Sharif-Askari, Saheb Sharif-Askari, Ali Hussain Alsayed, Alsafar et al., Vitamin D Enhances Type I IFN Signaling in COVID-19 Patients, Sci. Rep, doi:10.1038/s41598-022-22307-9
Ismailova, White, Vitamin, Infections and Immunity, Rev. Endocr. Metab. Disord, doi:10.1007/s11154-021-09679-5
Kaufman, Niles, Kroll, Bi, Holick, SARS-CoV-2 Positivity Rates Associated with Circulating 25-Hydroxyvitamin D Levels, PLoS ONE, doi:10.1371/journal.pone.0239252
Kulkarni, Gunnarsson, Yi, Gudmundsdottir, Sigurjonsson et al., Glucocorticoid Dexamethasone Down-Regulates Basal and Vitamin D3 Induced Cathelicidin Expression in Human Monocytes and Bronchial Epithelial Cell Line, Immunobiology, doi:10.1016/j.imbio.2015.09.001
Li, Hilgenfeld, Whitley, De Clercq, Therapeutic Strategies for COVID-19: Progress and Lessons Learned, Nat. Rev. Drug Discov, doi:10.1038/s41573-023-00672-y
Liu, Li, Xu, Wu, Luo et al., Prognostic Value of Interleukin-6, C-Reactive Protein, and Procalcitonin in Patients with COVID-19, J. Clin. Virol, doi:10.1016/j.jcv.2020.104370
Liu, Zhang, Dong, Li, Xu et al., Corticosteroid Treatment in Severe COVID-19 Patients with Acute Respiratory Distress Syndrome, J. Clin. Investig, doi:10.1172/JCI140617
Loucera, Peña-Chilet, Esteban-Medina, Muñoyerro-Muñiz, Villegas et al., Real World Evidence of Calcifediol or Vitamin D Prescription and Mortality Rate of COVID-19 in a Retrospective Cohort of Hospitalized Andalusian Patients, Sci. Rep, doi:10.1038/s41598-021-02701-5
Lugg, Thickett, The Role of Vitamin D in COVID-19, doi:10.1016/B978-0-323-91338-6.00049-5
Maghbooli, Sahraian, Jamalimoghadamsiahkali, Asadi, Zarei et al., Treatment with 25-Hydroxyvitamin D 3 (Calcifediol) Is Associated with a Reduction in the Blood Neutrophil-to-Lymphocyte Ratio Marker of Disease Severity in Hospitalized Patients with COVID-19: A Pilot Multicenter, Randomized, Placebo-Controlled, Double-Bli, Endocr. Pract, doi:10.1016/j.eprac.2021.09.016
Marcellini, Swieboda, Guedán, Farrow, Casolari et al., Glucocorticoids Impair Type I IFN Signalling and Enhance Rhinovirus Replication, Eur. J. Pharmacol, doi:10.1016/j.ejphar.2020.173839
Matsuyama, Kubli, Yoshinaga, Pfeffer, Mak, An Aberrant STAT Pathway Is Central to COVID-19, Cell Death Differ, doi:10.1038/s41418-020-00633-7
Mehta, Mcauley, Brown, Sanchez, Tattersall et al., Consider Cytokine Storm Syndromes and Immunosuppression, Lancet, doi:10.1016/S0140-6736(20)30628-0
Meltzer, Best, Zhang, Vokes, Arora et al., Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2020.19722
Merad, Blish, Sallusto, Iwasaki, The Immunology and Immunopathology of COVID-19, Science, doi:10.1126/science.abm8108
Mizuno, Suzuki, Takahashi, Imai, Itagaki et al., The Effect of Baricitinib and Corticosteroid Compared to That of Corticosteroid Monotherapy in Severely and Critically Ill Patients with COVID-19: A Japanese Multicenter Inpatient Database Study, J. Infect. Chemother, doi:10.1016/j.jiac.2024.09.020
Nogues, Ovejero, Pineda-Moncusí, Bouillon, Arenas et al., Calcifediol Treatment and COVID-19-Related Outcomes, J. Clin. Endocrinol. Metab, doi:10.1210/clinem/dgab405
Oristrell, Oliva, Casado, Subirana, Domínguez et al., Vitamin D Supplementation and COVID-19 Risk: A Population-Based, Cohort Study, J. Endocrinol. Investig, doi:10.1007/s40618-021-01639-9
Pagano, Gauvreau, Principles of Biostatistics, doi:10.1201/9780429489624
Plaçais, Richier, Noël, Lacombe, Mariette et al., Immune Interventions in COVID-19: A Matter of Time?, Mucosal Immunol, doi:10.1038/s41385-021-00464-w
Pérez-Alba, Nuzzolo-Shihadeh, Aguirre-García, Espinosa-Mora, Lecona-Garcia et al., Baricitinib plus Dexamethasone Compared to Dexamethasone for the Treatment of Severe COVID-19 Pneumonia: A Retrospective Analysis, J. Microbiol. Immunol. Infect, doi:10.1016/j.jmii.2021.05.009
Quesada-Gomez, Bouillon, Is Calcifediol Better than Cholecalciferol for Vitamin D Supplementation?, Osteoporos. Int, doi:10.1007/s00198-018-4520-y
Quesada-Gomez, Entrenas-Castillo, Bouillon, Vitamin D Receptor Stimulation to Reduce Acute Respiratory Distress Syndrome (ARDS) in Patients with Coronavirus SARS-CoV-2 Infections: Revised Ms SBMB 2020_166, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2020.105719
Quesada-Gomez, Lopez-Miranda, Entrenas-Castillo, Casado-Díaz, Nogues et al., Vitamin D Endocrine System and COVID-19, Nutrients, doi:10.3390/nu14132716
Raharinirina, Gubela, Börnigen, Smith, Oh et al., SARS-CoV-2 Evolution on a Dynamic Immune Landscape, Nature, doi:10.1038/s41586-024-08477-8
Ranabhat, Jakovljevic, Kim, Simkhada, COVID-19 Pandemic: An Opportunity for Universal Health Coverage, Front. Public. Health, doi:10.3389/fpubh.2021.673542
Rysz, Al-Saadi, Sjöström, Farm, Campoccia Jalde et al., COVID-19 Pathophysiology May Be Driven by an Imbalance in the Renin-Angiotensin-Aldosterone System, Nat. Commun, doi:10.1038/s41467-021-22713-z
Sarzani, Spannella, Giulietti, Di Pentima, Giordano et al., Possible Harm from Glucocorticoid Drugs Misuse in the Early Phase of SARS-CoV-2 Infection: A Narrative Review of the Evidence, Intern. Emerg. Med, doi:10.1007/s11739-021-02860-3
Smolders, Van Den Ouweland, Geven, Pickkers, Kox, Letter to the Editor: Vitamin D Deficiency in COVID-19: Mixing up Cause and Consequence, Metabolism, doi:10.1016/j.metabol.2020.154434
Stebbing, Nievas, Falcone, Youhanna, Richardson et al., JAK Inhibition Reduces SARS-CoV-2 Liver Infectivity and Modulates Inflammatory Responses to Reduce Morbidity and Mortality, Sci. Adv, doi:10.1126/sciadv.abe4724
Stebbing, Phelan, Griffin, Tucker, Oechsle et al., COVID-19: Combining Antiviral and Anti-Inflammatory Treatments, Lancet Infect. Dis, doi:10.1016/S1473-3099(20)30132-8
Tay, Poh, Rénia, Macary, Ng, The Trinity of COVID-19: Immunity, Inflammation and Intervention, Nat. Rev. Immunol, doi:10.1038/s41577-020-0311-8
Wang, Joshi, Leopold, Jackson, Christensen et al., Association of Vitamin D Deficiency with COVID-19 Infection Severity: Systematic Review and Meta-analysis, Clin. Endocrinol, doi:10.1111/cen.14540
Wang, Yang, Chen, Guo, Liu et al., The Proportion and Effect of Corticosteroid Therapy in Patients with COVID-19 Infection: A Systematic Review and Meta-Analysis, PLoS ONE, doi:10.1371/journal.pone.0249481
Wang, Yang, Li, Huang, Jiang et al., Specific Cytokines in the Inflammatory Cytokine Storm of Patients with COVID-19-Associated Acute Respiratory Distress Syndrome and Extrapulmonary Multiple-Organ Dysfunction, Virol. J, doi:10.1186/s12985-021-01588-y
Wiersinga, Rhodes, Cheng, Peacock, Prescott et al., Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review, JAMA, doi:10.1001/jama.2020.12839
Young, Clyne, Chapman, Endocrine Aspects of ACE2 Regulation: RAAS, Steroid Hormones and SARS-CoV-2, J. Endocrinol, doi:10.1530/JOE-20-0260
Yuan, Ma, Xie, Li, Su et al., The Role of Cell Death in SARS-CoV-2 Infection, Signal Transduct. Target. Ther, doi:10.1038/s41392-023-01580-8
Zhao, Yu, Mao, He, Huang et al., Effect of 25-Hydroxyvitamin D 3 on Rotavirus Replication and Gene Expressions of RIG-I Signalling Molecule in Porcine Rotavirus-Infected IPEC-J2 Cells, Arch. Anim. Nutr, doi:10.1080/1745039X.2015.1034522
