Efficacy and safety of vitamin D supplementation in hospitalized COVID-19 pediatric patients: A randomized controlled trial
et al., Frontiers in Pediatrics, doi:10.3389/fped.2022.943529, NCT04502667, Jul 2022
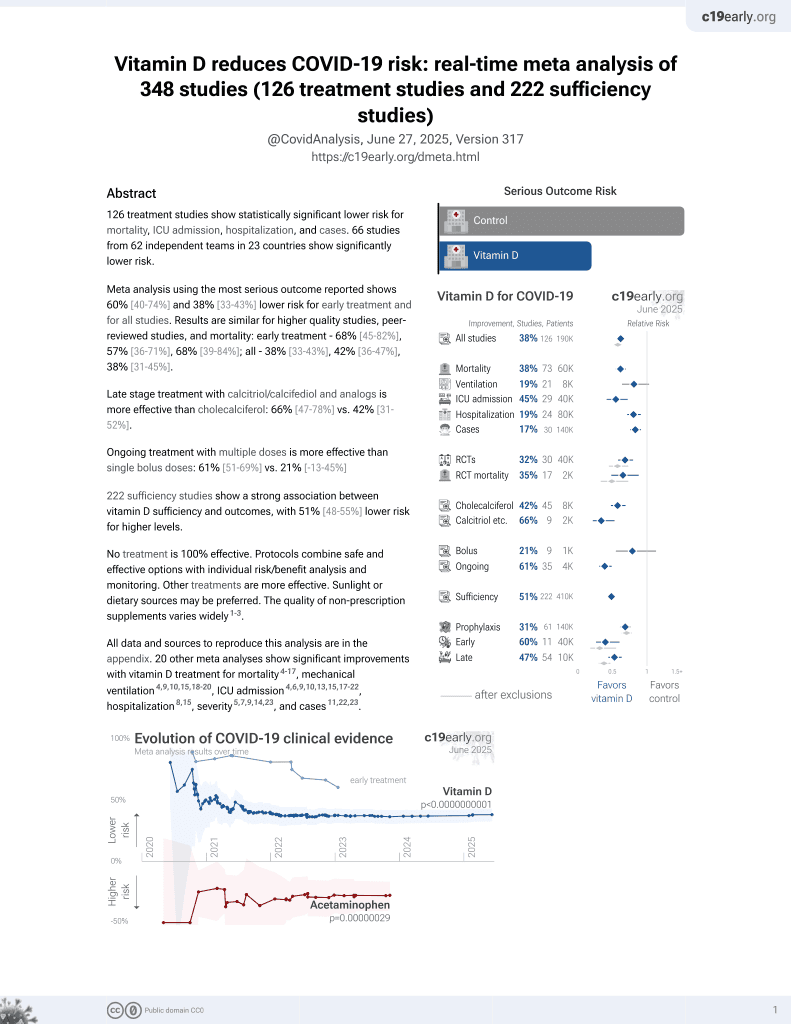
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 136 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 45 hospitalized high-risk pediatric patients requiring supplemental oxygen in Mexico, showing lower mortality, ventilation, and intensive care with vitamin D treatment, however there were less severe and critical cases at baseline in the treatment group.
Cholecalciferol was used in this study.
Meta-analysis shows that late stage treatment with calcitriol / calcifediol (or
paricalcitol, alfacalcidol, etc.) is more effective than cholecalciferol: 66% [47‑78%] lower risk vs. 44% [33‑53%] lower risk.
Cholecalciferol requires two hydroxylation steps to become activated - first
in the liver to calcifediol, then in the kidney to calcitriol. Calcitriol,
paricalcitol, and alfacalcidol are active vitamin D analogs that do not
require conversion. This allows them to have more rapid onset of action
compared to cholecalciferol. The time delay for cholecalciferol to increase
serum calcifediol levels can be 2-3 days, and the delay for converting
calcifediol to active calcitriol can be up to 7 days.
This is the 24th of 40 COVID-19 RCTs for vitamin D, which collectively show efficacy with p=0.0000001.
This is the 94th of 136 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
This study is excluded in the after exclusion results of meta-analysis:
randomization resulted in significant baseline differences that were not adjusted for.
|
risk of death, 79.2% lower, RR 0.21, p = 0.11, treatment 1 of 20 (5.0%), control 6 of 25 (24.0%), NNT 5.3.
|
|
risk of mechanical ventilation, 72.2% lower, RR 0.28, p = 0.08, treatment 2 of 20 (10.0%), control 9 of 25 (36.0%), NNT 3.8.
|
|
risk of ICU admission, 73.2% lower, RR 0.27, p = 0.006, treatment 3 of 20 (15.0%), control 14 of 25 (56.0%), NNT 2.4.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Zurita-Cruz et al., 25 Jul 2022, Single Blind Randomized Controlled Trial, Mexico, peer-reviewed, median age 12.0, 7 authors, study period 24 March, 2020 - 31 March, 2021, dosage 2,000IU daily, daily, 1,000IU for children <1 year, trial NCT04502667 (history).
Contact: mirandaguadalupe2707@yahoo.com, guadalupe.mirandan@imss.gob.mx.
E cacy and safety of vitamin D supplementation in hospitalized COVID-pediatric patients: A randomized controlled trial
Novales G ( ) E cacy and safety of vitamin D supplementation in hospitalized COVID-pediatric patients: A randomized controlled trial.
Ethics statement The studies involving human participants were reviewed and approved by Comité Local de Ética en Investigación number 3603, authorization number R-2020-3603-20. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions JZ-C, MV-K, ML-A, and GM-N: study design. JF-T, IP-O, BL-M, and GM-N: collection, analysis, and interpretation of data. JZ-C, GM-N, and MV-K: manuscript preparation and final approval. All the authors have contributed to the article and approved the submitted version of the manuscript.
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agarwal, Rochwerg, Lamontagne, Siemieniuk, Agoritsas et al., A living WHO guideline on drugs for COVID-19, BMJ, doi:10.1136/bmj.m3379
Banchereau, Steinman, Dendritic cells and the control of immunity, Nature, doi:10.1038/32588
Castillo, Costa, Barrios, Díaz, Miranda et al., Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study, J Steroid Biochem Mol Biol, doi:10.1016/j.jsbmb.2020.105751
Chiodini, Gatti, Soranna, Merlotti, Mingiano et al., The effect of high-dose parenteral vitamin D3 on COVID-19-related inhospital mortality in critical COVID-19 patients during intensive care unit admission: an observational cohort study, Front Public Health, doi:10.1038/s41430-021-00984-5
Esposito, Marchetti, Lanari, Caramelli, Fanti et al., COVID-19 management in the pediatric age: consensus document of the COVID-19 working group in paediatrics of the Emilia-Romagna region (RE-CO-Ped), Italy, Int J Environ Res Public Health, doi:10.3390/ijerph18083919
González-García, Castilla-Peón, Santos, Bustamante, Hibert, COVID-19 incidence and mortality by age strata and comorbidities in Mexico city: a focus in the pediatric population, Front Public Health, doi:10.1038/nature06175
Holick, Binkley, Bischoff-Ferrari, Gordon, Hanley et al., Evaluation, treatment, and prevention of Vitamin D deficiency: and endocrine society clinical practice guideline, J Clin Endocrinol Metabol, doi:10.1210/jc.2011-0385
Kalinina, Vitamin D intake may reduce SARS-CoV-2 infection morbidity in health care workers, Nutrients, doi:10.3390/nu14030505
Kang, Jeong, Suh, Ahn, The impact of the Coronavirus disease-2019 pandemic on childhood obesity and vitamin D status, J Korean Med Sci, doi:10.3346/jkms.2021.36.e21
Kumar, Rathi, Haq, Wimalawansa, Sharma, Putative roles of vitamin D in modulating immune response and immunopathology associated with COVID-19, Virus Res, doi:10.1016/j.virusres.2020.198235
Liu, He, Liu, Li, Shi, Children with COVID-19 behaving milder may challenge the public policies: a systematic review and meta-analysis, BMC Pediatr, doi:10.1186/s12887-020-02316-1
Liu, Sun, Wang, Zhang, Zhao et al., Low vitamin D status is associated with coronavirus disease 2019 outcomes: a systematic review and meta-analysis, Int J Infect Dis, doi:10.1186/s12879-021-06281-7
Mailhot, White, Vitamin D and immunity in infants and children, Nutrients, doi:10.3390/nu12051233
Meoli, Muggli, Lava, Bianchetti, Agostoni et al., Vitamin D status in adolescents during COVID-19 pandemic: a cross-sectional comparative study, Epidemiol Infect, doi:10.1017/S0950268821001825
Murai, Fernandes, Sales, Pinto, Goessler et al., Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2020.26848
Nogues, Ovejero, Pineda-Moncusí, Bouillon, Arenas et al., Calcifediol treatment and COVID-19-related outcomes, J Clin Endocrinol Metab, doi:10.1210/clinem/dgab405
Noriega, Savelkoul, Vitamin D: a potential mitigation tool for the endemic stage of the COVID-19 pandemic?, Front Public Health, doi:10.3389/fpubh.2022.888168
Oscanoa, Amado, Vidal, Laird, Ghashut et al., The relationship between the severity and mortality of SARS-CoV-2 infection and 25-hydroxyvitamin D concentration -a metaanalysis, Expert Rev Anti Infect Ther, doi:10.1101/2021.08.22.21262216
Penna, Amuchastegui, Giarratana, Daniel, Vulcano et al., 1, 25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells, J Immunol, doi:10.4049/jimmunol.178.1.145
Petrelli, Luciani, Perego, Dognini, Colombelli et al., Therapeutic and prognostic role of vitamin D for COVID-19 infection: a systematic review and meta-analysis of 43 observational studies, J Steroid Biochem Mol Biol, doi:10.1016/j.jsbmb.2021.105883
Ramirez-Sandoval, Vj, Paz-Cortés, Santillan-Ceron, Hernandez-Jimenez et al., Very low vitamin D levels are a strong independent predictor of mortality in hospitalized patients with severe COVID-19, Arch Med Res, doi:10.3390/nu13061990
Rastogi, Bhansali, Khare, Suri, Yaddanapudi et al., Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study), Postgrad Med J, doi:10.1136/postgradmedj-2020-139065
Shah, Varna, Pandya, Saxena, Low vitamin D levels and prognosis in a COVID-19 pediatric population: a systematic review, QJM, doi:10.1093/qjmed/hcab202
Simon, Hollander, Mcmichael, Evolution of the immune system in humans from infancy to old age, Proc Biol Sci, doi:10.1098/rspb.2014.3085
Somnath, Biswal, Chandrasekaran, Jagadisan, Bobby et al., Vitamin D supplementation for the prevention of childhood acute respiratory infections: a systematic review of randomised controlled trials, Clin Nutr ESPEN, doi:10.1017/S000711451500207X
Tessa, Motisi, Marseglia, Cardinale, Licari et al., Use of remdesivir in children with COVID-19 infection: a quick narrative review, Acta Biomed, doi:10.23750/abm.v92iS7.12396
Urashima, Segawa, Okazaki, Kuriharam, Wada et al., Reduced primary care respiratory infection visits following pregnancy and infancy vitamin D supplementation: a randomised controlled trial, Am J Clin Nutr, doi:10.1111/apa.12819
Van Den Ouweland, Beijers, Demacker, Van Daal, Measurement of 25-OH-vitamin D in human serum using liquid chromatography tandemmass spectrometry with comparison to radioimmunoassay and automated immunoassay, J Chromatogr B Analyt Technol Life Sci, doi:10.1016/j.jchromb.2010.03.035
Vecchio, Treatment of children with COVID-19: update of the Italian society of pediatric infectious diseases position paper, Ital J Pediatr, doi:10.1186/s13052-021-01132-2
Veldman, Cantorna, Deluca, Expression of 1,25-dihydroxyvitamin D3 receptor in the immune system, Arch Biochem Biophys, doi:10.1006/abbi.1999.1605
Venturini, Montagnani, Garazzino, Donà, Pierantoni et al., None
Wang, Nestel, Bourdeau, Nagai, Wang et al., A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections, Epidemiol Infect, doi:10.1017/S0950268809002404
Wang, Zhao, Tang, Wang, Shi et al., Potentially effective drugs for the treatment of COVID-19 or MIS-C in children: a systematic review, Eur J Pediatr, doi:10.1007/s00431-022-04388-w
Wrapp, Wang, Corbett, Goldsmith, Hsieh et al., Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation, Science, doi:10.1126/science.abb2507
DOI record:
{
"DOI": "10.3389/fped.2022.943529",
"ISSN": [
"2296-2360"
],
"URL": "http://dx.doi.org/10.3389/fped.2022.943529",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p>Some studies suggested that adequate levels of vitamin D (VD) decrease the risk of severe COVID-19. Information about the effectiveness of VD supplementation in children is scarce.</jats:p></jats:sec><jats:sec><jats:title>Objective</jats:title><jats:p>To assess the efficacy and safety of VD supplementation compared to the standard of care in hospitalized children with COVID-19.</jats:p></jats:sec><jats:sec><jats:title>Patients and methods</jats:title><jats:p>An open-label randomized controlled single-blind clinical trial was carried out. We included patients from 1 month to 17 years, with moderate COVID-19, who required hospitalization and supplemental oxygen. They were randomized into two groups: the VD group, which received doses of 1,000 (children &lt; 1 year) or 2,000 IU/day (from 1 to 17 years) and the group without VD (control). The outcome variables were the progression of oxygen requirement, the development of complications, and death.</jats:p></jats:sec><jats:sec><jats:title>Statistical analysis</jats:title><jats:p>For comparison between groups, we used the chi-squared test or Fisher's exact test and the Mann–Whitney <jats:italic>U</jats:italic> test. Absolute risk reduction (ARR) and the number needed to treat (NNT) were calculated. <jats:italic>p</jats:italic> ≤ 0.05 was considered statistically significant.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>From 24 March 2020 to 31 March 2021, 87 patients were eligible to participate in the trial; 45 patients were randomized: 20 to the VD group and 25 to the control group. There was no difference in general characteristics at baseline, including serum VD levels (median 13.8 ng/ml in the VD group and 11.4 ng/ml in the control group).</jats:p></jats:sec><jats:sec><jats:title>Outcomes</jats:title><jats:p>2/20 (10%) in the VD group vs. 9/25 (36%) in the control group progressed to a superior ventilation modality (<jats:italic>p</jats:italic> = 0.10); one patient in the VD group died (5%) compared to 6 (24%) patients in the control group (<jats:italic>p</jats:italic> = 0.23). ARR was 26% (95% CI 8.8 to 60.2%) and NNT was 3 (2 to 11) for progression and ARR was 19% (95% CI −3.9 to 42.8%) and NNT was 6 (2 to 26) for death. None of the patients receiving VD had adverse effects. The trial was stopped for ethical reasons; since after receiving the results of the basal VD values, none of the patients had normal levels.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>In this trial, VD supplementation in pediatric patients seems to decrease the risk of COVID-19 progression and death. More studies are needed to confirm these findings.</jats:p></jats:sec><jats:sec><jats:title>Clinical Trial Registration</jats:title><jats:p>This protocol was registered on <jats:ext-link>ClinicalTrials.gov</jats:ext-link> with the registration number NCT04502667.</jats:p></jats:sec>",
"alternative-id": [
"10.3389/fped.2022.943529"
],
"author": [
{
"affiliation": [],
"family": "Zurita-Cruz",
"given": "Jessie",
"sequence": "first"
},
{
"affiliation": [],
"family": "Fonseca-Tenorio",
"given": "Jeffry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Villasís-Keever",
"given": "Miguel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "López-Alarcón",
"given": "Mardia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parra-Ortega",
"given": "Israel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "López-Martínez",
"given": "Briceida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Miranda-Novales",
"given": "Guadalupe",
"sequence": "additional"
}
],
"container-title": "Frontiers in Pediatrics",
"container-title-short": "Front. Pediatr.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2022,
7,
25
]
],
"date-time": "2022-07-25T06:26:07Z",
"timestamp": 1658730367000
},
"deposited": {
"date-parts": [
[
2022,
7,
25
]
],
"date-time": "2022-07-25T06:26:11Z",
"timestamp": 1658730371000
},
"indexed": {
"date-parts": [
[
2022,
7,
25
]
],
"date-time": "2022-07-25T06:42:20Z",
"timestamp": 1658731340098
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
7,
25
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
25
]
],
"date-time": "2022-07-25T00:00:00Z",
"timestamp": 1658707200000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fped.2022.943529/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2022,
7,
25
]
]
},
"published-online": {
"date-parts": [
[
2022,
7,
25
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"key": "B1",
"unstructured": "Comité/Grupo de Pediatría Basada en la Evidencia de la AEP y AEPap2022"
},
{
"DOI": "10.1186/s13052-021-01132-2",
"article-title": "Treatment of children with COVID-19: update of the Italian society of pediatric infectious diseases position paper",
"author": "Venturini",
"doi-asserted-by": "publisher",
"first-page": "199",
"journal-title": "Ital J Pediatr.",
"key": "B2",
"volume": "47",
"year": "2021"
},
{
"DOI": "10.1007/s00431-022-04388-w",
"article-title": "Potentially effective drugs for the treatment of COVID-19 or MIS-C in children: a systematic review",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "2135",
"journal-title": "Eur J Pediatr",
"key": "B3",
"volume": "181",
"year": "2022"
},
{
"DOI": "10.1136/bmj.m3379",
"article-title": "A living WHO guideline on drugs for COVID-19",
"author": "Agarwal",
"doi-asserted-by": "publisher",
"first-page": "m3379",
"journal-title": "BMJ",
"key": "B4",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.23750/abm.v92iS7.12396",
"article-title": "Use of remdesivir in children with COVID-19 infection: a quick narrative review",
"author": "La Tessa",
"doi-asserted-by": "publisher",
"first-page": "e2021524",
"journal-title": "Acta Biomed.",
"key": "B5",
"volume": "92",
"year": "2021"
},
{
"DOI": "10.1098/rspb.2014.3085",
"article-title": "Evolution of the immune system in humans from infancy to old age",
"author": "Simon",
"doi-asserted-by": "publisher",
"first-page": "20143085",
"journal-title": "Proc Biol Sci.",
"key": "B6",
"volume": "282",
"year": "2015"
},
{
"DOI": "10.1126/science.abb2507",
"article-title": "Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation",
"author": "Wrapp",
"doi-asserted-by": "publisher",
"first-page": "1260",
"journal-title": "Science.",
"key": "B7",
"volume": "267",
"year": "2020"
},
{
"DOI": "10.1186/s12887-020-02316-1",
"article-title": "Children with COVID-19 behaving milder may challenge the public policies: a systematic review and meta-analysis",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "410",
"journal-title": "BMC Pediatr.",
"key": "B8",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.3389/fpubh.2021.738423",
"article-title": "COVID-19 incidence and mortality by age strata and comorbidities in Mexico city: a focus in the pediatric population",
"author": "González-García",
"doi-asserted-by": "publisher",
"first-page": "738423",
"journal-title": "Front Public Health",
"key": "B9",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1111/j.1365-2265.2011.04261.x",
"article-title": "An update on vitamin D and human immunity",
"author": "Hewison",
"doi-asserted-by": "publisher",
"first-page": "315",
"journal-title": "Clin Endocrinol.",
"key": "B10",
"volume": "76",
"year": "2012"
},
{
"DOI": "10.1038/nature06175",
"article-title": "Taking dendritic cells into medicine",
"author": "Steinman",
"doi-asserted-by": "publisher",
"first-page": "419",
"journal-title": "Nature.",
"key": "B11",
"volume": "449",
"year": "2007"
},
{
"DOI": "10.1038/32588",
"article-title": "Dendritic cells and the control of immunity",
"author": "Banchereau",
"doi-asserted-by": "publisher",
"first-page": "245",
"journal-title": "Nature.",
"key": "B12",
"volume": "392",
"year": "1998"
},
{
"DOI": "10.4049/jimmunol.178.1.145",
"article-title": "1, 25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells",
"author": "Penna",
"doi-asserted-by": "publisher",
"first-page": "145",
"journal-title": "J Immunol.",
"key": "B13",
"volume": "178",
"year": "2007"
},
{
"DOI": "10.1006/abbi.1999.1605",
"article-title": "Expression of 1,25-dihydroxyvitamin D3 receptor in the immune system",
"author": "Veldman",
"doi-asserted-by": "publisher",
"first-page": "334",
"journal-title": "Arch Biochem Biophys",
"key": "B14",
"volume": "374",
"year": "2000"
},
{
"DOI": "10.3390/nu12051233",
"article-title": "Vitamin D and immunity in infants and children",
"author": "Mailhot",
"doi-asserted-by": "publisher",
"first-page": "1233",
"journal-title": "Nutrients.",
"key": "B15",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.4049/jimmunol.173.5.2909",
"article-title": "Cutting edge: 1, 25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "2909",
"journal-title": "J Immunol.",
"key": "B16",
"volume": "173",
"year": "2004"
},
{
"DOI": "10.1371/journal.pone.0025333",
"article-title": "Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37",
"author": "Barlow",
"doi-asserted-by": "publisher",
"first-page": "e25333",
"journal-title": "PLoS ONE",
"key": "B17",
"volume": "6",
"year": "2011"
},
{
"DOI": "10.1017/S0950268809002404",
"article-title": "A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections",
"author": "Li-Ng",
"doi-asserted-by": "publisher",
"first-page": "1396",
"journal-title": "Epidemiol Infect.",
"key": "B18",
"volume": "137",
"year": "2009"
},
{
"DOI": "10.3945/ajcn.2009.29094",
"article-title": "Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren",
"author": "Urashima",
"doi-asserted-by": "publisher",
"first-page": "1255",
"journal-title": "Am J Clin Nutr",
"key": "B19",
"volume": "91",
"year": "2010"
},
{
"DOI": "10.1111/apa.12819",
"article-title": "Reduced primary care respiratory infection visits following pregnancy and infancy vitamin D supplementation: a randomised controlled trial",
"author": "Grant",
"doi-asserted-by": "publisher",
"first-page": "396",
"journal-title": "Acta Paediatr.",
"key": "B20",
"volume": "104",
"year": "2015"
},
{
"DOI": "10.1016/j.clnesp.2017.02.003",
"article-title": "Therapeutic effect of vitamin D in acute lower respiratory infection: a randomized controlled trial",
"author": "Somnath",
"doi-asserted-by": "publisher",
"first-page": "24",
"journal-title": "Clin Nutr ESPEN.",
"key": "B21",
"volume": "20",
"year": "2017"
},
{
"DOI": "10.1017/S000711451500207X",
"article-title": "Vitamin D supplementation for the prevention of childhood acute respiratory infections: a systematic review of randomised controlled trials",
"author": "Xiao",
"doi-asserted-by": "publisher",
"first-page": "1026",
"journal-title": "Br J Nutr.",
"key": "B22",
"volume": "114",
"year": "2015"
},
{
"DOI": "10.1016/j.jsbmb.2021.105883",
"article-title": "Therapeutic and prognostic role of vitamin D for COVID-19 infection: a systematic review and meta-analysis of 43 observational studies",
"author": "Petrelli",
"doi-asserted-by": "publisher",
"first-page": "105883",
"journal-title": "J Steroid Biochem Mol Biol.",
"key": "B23",
"volume": "211",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2020.12.077",
"article-title": "Low vitamin D status is associated with coronavirus disease 2019 outcomes: a systematic review and meta-analysis",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "58",
"journal-title": "Int J Infect Dis.",
"key": "B24",
"volume": "104",
"year": "2021"
},
{
"DOI": "10.1186/s12879-021-06281-7",
"article-title": "Vitamin D and COVID-19 severity and related mortality: a prospective study in Italy",
"author": "Campi",
"doi-asserted-by": "publisher",
"first-page": "566",
"journal-title": "BMC Infect Dis",
"key": "B25",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.3389/fpubh.2021.736665",
"article-title": "Vitamin D status and SARS-CoV-2 infection and COVID-19 clinical outcomes",
"author": "Chiodini",
"doi-asserted-by": "publisher",
"first-page": "736665",
"journal-title": "Front Public Health.",
"key": "B26",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1038/s41430-021-00984-5",
"article-title": "The effect of high-dose parenteral vitamin D3 on COVID-19-related inhospital mortality in critical COVID-19 patients during intensive care unit admission: an observational cohort study",
"author": "Güven",
"doi-asserted-by": "publisher",
"first-page": "1383",
"journal-title": "Eur J Clin Nutr.",
"key": "B27",
"volume": "75",
"year": "2021"
},
{
"DOI": "10.1016/j.jsbmb.2020.105751",
"article-title": "Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study",
"author": "Entrenas Castillo",
"doi-asserted-by": "publisher",
"first-page": "105751",
"journal-title": "J Steroid Biochem Mol Biol.",
"key": "B28",
"volume": "203",
"year": "2020"
},
{
"DOI": "10.1136/postgradmedj-2020-139065",
"article-title": "Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study)",
"author": "Rastogi",
"doi-asserted-by": "publisher",
"first-page": "87",
"journal-title": "Postgrad Med J",
"key": "B29",
"volume": "98",
"year": "2022"
},
{
"DOI": "10.3390/ijerph18083919",
"article-title": "COVID-19 management in the pediatric age: consensus document of the COVID-19 working group in paediatrics of the Emilia-Romagna region (RE-CO-Ped), Italy",
"author": "Esposito",
"doi-asserted-by": "publisher",
"first-page": "3919",
"journal-title": "Int J Environ Res Public Health.",
"key": "B30",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1016/j.jchromb.2010.03.035",
"article-title": "Measurement of 25-OH-vitamin D in human serum using liquid chromatography tandem-mass spectrometry with comparison to radioimmunoassay and automated immunoassay",
"author": "Van den Ouweland",
"doi-asserted-by": "publisher",
"first-page": "1163",
"journal-title": "J Chromatogr B Analyt Technol Life Sci.",
"key": "B31",
"volume": "878",
"year": "2010"
},
{
"key": "B32",
"unstructured": ""
},
{
"DOI": "10.1016/j.arcmed.2021.09.006",
"article-title": "Very low vitamin D levels are a strong independent predictor of mortality in hospitalized patients with severe COVID-19",
"author": "Ramirez-Sandoval",
"doi-asserted-by": "publisher",
"first-page": "215",
"journal-title": "Arch Med Res.",
"key": "B33",
"volume": "53",
"year": "2022"
},
{
"DOI": "10.3390/nu13061990",
"article-title": "The impact of COVID-19 pandemic during 2020-2021 on the vitamin D serum levels in the paediatric population in Warsaw, Poland",
"author": "Rustecka",
"doi-asserted-by": "publisher",
"first-page": "1990",
"journal-title": "Nutrients.",
"key": "B34",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.3346/jkms.2021.36.e21",
"article-title": "The impact of the Coronavirus disease-2019 pandemic on childhood obesity and vitamin D status",
"author": "Kang",
"doi-asserted-by": "publisher",
"first-page": "e21",
"journal-title": "J Korean Med Sci.",
"key": "B35",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.3390/nu13051467",
"article-title": "Vitamin D status in adolescents during COVID-19 pandemic: a cross-sectional comparative study",
"author": "Meoli",
"doi-asserted-by": "publisher",
"first-page": "1467",
"journal-title": "Nutrients.",
"key": "B36",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1017/S0950268821001825",
"article-title": "Vitamin D levels in children with COVID-19: a report from Turkey",
"author": "Alpcan",
"doi-asserted-by": "publisher",
"first-page": "e180",
"journal-title": "Epidemiol Infect.",
"key": "B37",
"volume": "149",
"year": "2021"
},
{
"DOI": "10.1016/j.virusres.2020.198235",
"article-title": "Putative roles of vitamin D in modulating immune response and immunopathology associated with COVID-19",
"author": "Kumar",
"doi-asserted-by": "publisher",
"first-page": "198235",
"journal-title": "Virus Res.",
"key": "B38",
"volume": "292",
"year": "2021"
},
{
"DOI": "10.3390/nu14030505",
"article-title": "Vitamin D intake may reduce SARS-CoV-2 infection morbidity in health care workers",
"author": "Karonova",
"doi-asserted-by": "publisher",
"first-page": "505",
"journal-title": "Nutrients.",
"key": "B39",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1001/jama.2020.26848",
"article-title": "Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial",
"author": "Murai",
"doi-asserted-by": "publisher",
"first-page": "1053",
"journal-title": "JAMA.",
"key": "B40",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1210/clinem/dgab405",
"article-title": "Calcifediol treatment and COVID-19-related outcomes",
"author": "Nogues",
"doi-asserted-by": "publisher",
"first-page": "e4017",
"journal-title": "J Clin Endocrinol Metab.",
"key": "B41",
"volume": "106",
"year": "2021"
},
{
"DOI": "10.5603/ARM.a2021.0037",
"article-title": "The relationship between the severity and mortality of SARS-CoV-2 infection and 25-hydroxyvitamin D concentration - a metaanalysis",
"author": "Oscanoa",
"doi-asserted-by": "publisher",
"first-page": "145",
"journal-title": "Adv Respir Med.",
"key": "B42",
"volume": "89",
"year": "2021"
},
{
"DOI": "10.1101/2021.08.22.21262216",
"article-title": "COVID-19 and vitamin D (Co-VIVID study): a systematic review and meta-analysis of randomized controlled trials",
"author": "Varikasuvu",
"doi-asserted-by": "publisher",
"first-page": "907",
"journal-title": "Expert Rev Anti Infect Ther",
"key": "B43",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.1093/qjmed/hcab202",
"article-title": "Low vitamin D levels and prognosis in a COVID-19 pediatric population: a systematic review",
"author": "Shah",
"doi-asserted-by": "publisher",
"first-page": "447",
"journal-title": "QJM.",
"key": "B44",
"volume": "114",
"year": "2021"
},
{
"DOI": "10.1210/jc.2011-0385",
"article-title": "Evaluation, treatment, and prevention of Vitamin D deficiency: and endocrine society clinical practice guideline",
"author": "Holick",
"doi-asserted-by": "publisher",
"first-page": "1911",
"journal-title": "J Clin Endocrinol Metabol.",
"key": "B45",
"volume": "96",
"year": "2011"
},
{
"DOI": "10.3389/fpubh.2022.888168",
"article-title": "Vitamin D: a potential mitigation tool for the endemic stage of the COVID-19 pandemic?",
"author": "Briceno Noriega",
"doi-asserted-by": "publisher",
"journal-title": "Front Public Health.",
"key": "B46",
"volume": "10",
"year": "2022"
}
],
"reference-count": 46,
"references-count": 46,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fped.2022.943529/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pediatrics, Perinatology and Child Health"
],
"subtitle": [],
"title": "Efficacy and safety of vitamin D supplementation in hospitalized COVID-19 pediatric patients: A randomized controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "10"
}