The effect of high-dose parenteral vitamin D3 on COVID-19-related inhospital mortality in critical COVID-19 patients during intensive care unit admission: an observational cohort study
et al., European Journal of Clinical Nutrition, doi:10.1038/s41430-021-00984-5, Jul 2021
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 135 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 175 ICU patients, 113 treated with a single dose of 300,000IU intramuscular cholecalciferol, showing lower mortality with treatment, but not reaching statistical significance. Calcifediol or calcitriol, which avoids several days delay in conversion, may be more successful, especially with this very late stage usage.
Cholecalciferol was used in this study.
Meta-analysis shows that late stage treatment with calcitriol / calcifediol (or
paricalcitol, alfacalcidol, etc.) is more effective than cholecalciferol: 66% [47‑78%] lower risk vs. 45% [34‑54%] lower risk.
Cholecalciferol requires two hydroxylation steps to become activated - first
in the liver to calcifediol, then in the kidney to calcitriol. Calcitriol,
paricalcitol, and alfacalcidol are active vitamin D analogs that do not
require conversion. This allows them to have more rapid onset of action
compared to cholecalciferol. The time delay for cholecalciferol to increase
serum calcifediol levels can be 2-3 days, and the delay for converting
calcifediol to active calcitriol can be up to 7 days.
Bolus treatment is less effective.
Pharmacokinetics and the potential side effects of high bolus doses suggest
that ongoing treatment spread over time is more appropriate.
Research has confirmed that lower dose regular treatment with vitamin D is more
effective than intermittent high-dose bolus treatment for various conditions,
including rickets and acute respiratory infections1,2. The biological mechanisms supporting these
findings involve the induction of enzymes such as 24-hydroxylase and
fibroblast growth factor 23 (FGF23) by high-dose bolus treatments. These
enzymes play roles in inactivating vitamin D, which can paradoxically reduce
levels of activated vitamin D and suppress its activation for extended periods
post-dosage. Evidence indicates that 24-hydroxylase activity may remain
elevated for several weeks following a bolus dose, leading to reduced levels
of the activated form of vitamin D. Additionally, FGF23 levels can increase
for at least three months after a large bolus dose, which also contributes to
the suppression of vitamin D activation1.
This is the 43rd of 135 COVID-19 controlled studies for vitamin D, which collectively show efficacy with p<0.0000000001.
40 studies are RCTs, which show efficacy with p=0.0000001.
This study is excluded in the after exclusion results of meta-analysis:
very late stage, ICU patients.
|
risk of death, 24.8% lower, RR 0.75, p = 0.32, treatment 43 of 113 (38.1%), control 30 of 62 (48.4%), NNT 9.7, odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Güven et al., 23 Jul 2021, retrospective, Turkey, peer-reviewed, 2 authors, dosage 300,000IU single dose.
The effect of high-dose parenteral vitamin D3 on COVID-19-related inhospital mortality in critical COVID-19 patients during intensive care unit admission: an observational cohort study
European Journal of Clinical Nutrition, doi:10.1038/s41430-021-00984-5
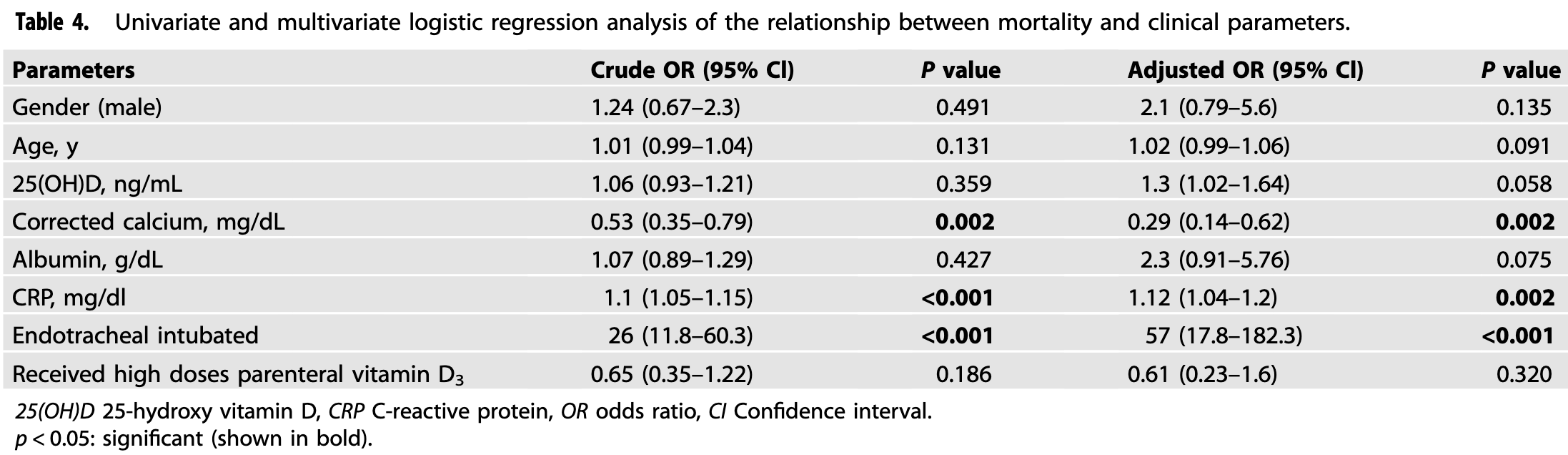
BACKGROUND: In many studies, vitamin D has been found to be low in COVID-19 patients. In this study, we aimed to investigate the relationship between clinical course and inhospital mortality with parenteral administration of high-dose vitamin D 3 within the first 24 h of admission to patients who were hospitalized in the intensive care unit (ICU) because of COVID-19 with vitamin D deficiency. METHODS: This study included 175 COVID-19 patients with vitamin D deficiency [25(OH) D <12 ng/mL] who were hospitalized in the ICU. Vitamin D 3 group (n = 113) included patients who received a single dose of 300,000 IU vitamin D3 intramuscularly. Vitamin D 3 was not administered to the control group (n = 62). RESULTS: Median C-reactive protein level was 10.8 mg/dL in the vitamin D 3 group and 10.6 mg/dL in the control group (p = 0.465). Thirty-nine percent (n = 44) of the patients in the vitamin D 3 group were intubated endotracheally, and 50% (n = 31) of the patients in the control group were intubated endotracheally (p = 0.157). Parenteral vitamin D 3 administration was not associated with inhospital mortality by multivariate logistic regression analysis. According to Kaplan-Meier survival analysis, the median survival time was 16 d in the vitamin D3 group and 17 d in the control group (log-rank test, p = 0.459). CONCLUSION: In this study, which was performed for the first time in the literature, it was observed that high-dose parenteral vitamin D 3 administration in critical COVID-19 patients with vitamin D deficiency during admission to the ICU did not reduce the need for intubation, length of hospital stay, and inhospital mortality.
COMPETING INTERESTS The authors declare no competing interests.
ADDITIONAL INFORMATION Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41430-021-00984-5. Correspondence and requests for materials should be addressed to M.G. Reprints and permission information is available at http://www.nature.com/ reprints Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Abraham, Dowling, Florentine, Can optimum solar radiation exposure or supplemented vitamin D intake reduce the severity of COVID-19 symptoms?, Int J Environ Res Public Health, doi:10.3390/ijerph18020740
Amrein, Schnedl, Holl, Riedl, Christopher et al., Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial, JAMA, doi:10.1001/jama.2014.13204
Asan, Üstündağ, Koca, Şimşek, Sayan et al., Do initial hematologic indices predict the severity of COVID-19 patients?, Turk J Med Sci, doi:10.3906/sag-2007-97
Atkins, Masoli, Delgado, Pilling, Kuo et al., Preexisting comorbidities predicting COVID-19 and mortality in the UK Biobank Community Cohort, J Gerontol A Biol Sci Med Sci, doi:10.1093/gerona/glaa183
Bilezikian, Bikle, Hewison, Castro, Formenti et al., MECHANISMS IN ENDOCRINOLOGY: vitamin D and COVID-19, Eur J Endocrinol, doi:10.1530/EJE-20-0665
Brockman-Schneider, Pickles, Gern, Effects of vitamin D on airway epithelial cell morphology and rhinovirus replication, PLoS ONE, doi:10.1371/journal.pone.0086755
Calder, Nutrition, immunity and COVID-19, BMJ Nutr Prev Health, doi:10.1136/bmjnph-2020-000085
Castillo, Costa, Barrios, Díaz, Miranda et al., Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study, J Steroid Biochem Mol Biol, doi:10.1016/j.jsbmb.2020.105751
Charoenngam, Holick, Immunologic effects of vitamin D on human health and disease, Nutrients, doi:10.3390/nu12072097
Chen, Zhang, Ge, Du, Deb et al., Vitamin D inhibits nuclear factor κ B activation by interacting with IκB kinase β protein, J Biol Chem, doi:10.1074/jbc.M113.467670
Conti, Ronconi, Caraffa, Gallenga, Ross et al., Induction of proinflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies, J Biol Regul Homeost Agents, doi:10.23812/CONTI-E
Ebadi, Montano-Loza, Perspective: improving vitamin D status in the management of COVID-19, Eur J Clin Nutr, doi:10.1038/s41430-020-0661-0
Fig, 2 Survival of the groups according to Kaplan-Meier analysis
Gombart, Borregaard, Koeffler, Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3, FASEB J, doi:10.1096/fj.04-3284com
Gonçalves, Gonçalves, Guarnieri, Risegato, Guimarães et al., Prevalence of obesity and hypovitaminosis D in elderly with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Clin Nutr ESPEN, doi:10.1016/j.clnesp.2020.10.008
Grant, Lahore, Mcdonnell, Baggerly, French et al., Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths, Nutrients, doi:10.3390/nu12040988
Greiller, Suri, Jolliffe, Kebadze, Hirsman et al., Vitamin D attenuates rhinovirus-induced expression of intercellular adhesion molecule-1 (ICAM-1) and platelet-activating factor receptor (PAFR) in respiratory epithelial cells, J Steroid Biochem Mol Biol, doi:10.1016/j.jsbmb.2018.11.013
Guan, Liang, Zhao, Liang, Chen et al., Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis, Eur Respir J, doi:10.1183/13993003.00547-2020
Güven, Gültekin, Could serum total cortisol level at admission predict mortality due to coronavirus disease 2019 in the intensive care unit? A prospective study, Sao Paulo Med J, doi:10.1590/1516-3180.2020.0722.R1.2302021
Han, Jones, Tangpricha, Brown, Brown et al., High dose vitamin D administration in ventilated intensive care unit patients: a pilot double blind randomized controlled trial, J Clin Transl Endocrinol, doi:10.1016/j.jcte.2016.04.004
Hastie, Pell, Sattar, Vitamin D and COVID-19 infection and mortality in UK Biobank, Eur J Nutr, doi:10.1007/s00394-020-02372-4
Hughes, Norton, Vitamin D and respiratory health, Clin Exp Immunol, doi:10.1111/j.1365-2249.2009.04001.x
Kaufman, Niles, Kroll, Bi, Holick, SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels, PLoS ONE, doi:10.1371/journal.pone.0239252
Maghbooli, Sahraian, Ebrahimi, Pazoki, Kafan et al., Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection, PLoS ONE, doi:10.1371/journal.pone.0239799
Martineau, Forouhi, Vitamin D for COVID-19: a case to answer?, Lancet Diabetes Endocrinol, doi:10.1016/S2213-8587(20)30268-0
Martineau, Jolliffe, Hooper, Greenberg, Aloia et al., Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data, BMJ, doi:10.1136/bmj.i6583
Meltzer, Best, Zhang, Vokes, Arora et al., Association of vitamin D status and other clinical characteristics with COVID-19 test results, JAMA Netw Open, doi:10.1001/jamanetworkopen.2020.19722
Merzon, Tworowski, Gorohovski, Vinker, Cohen et al., Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study, FEBS J, doi:10.1111/febs.15495
Mitchell, Vitamin-D and COVID-19: do deficient risk a poorer outcome?, Lancet Diabetes Endocrinol, doi:10.1016/S2213-8587(20)30183-2
Munshi, Hussein, Toraih, Elshazli, Jardak et al., Vitamin D insufficiency as a potential culprit in critical COVID-19 patients, J Med Virol, doi:10.1002/jmv.26360
Murai, Fernandes, Sales, Pinto, Goessler et al., Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2020.26848
Pekar, Worobey, Moshiri, Scheffler, Wertheim, Timing the SARS-CoV-2 index case in Hubei province, Science, doi:10.1126/science.abf8003
Prietl, Treiber, Pieber, Amrein, Vitamin D and immune function, Nutrients, doi:10.3390/nu5072502
Solmaz, Özçaylak, Alakuş, Kılıç, Kalın et al., Risk factors affecting ICU admission in COVID-19 patients; Could air temperature be an effective factor?, Int J Clin Pract, doi:10.1111/ijcp.13803
Tamer, Mesçi, Role of vitamin D in the immune system, Turkjem, doi:10.4274/Tjem.1938
Thomson, Hunter, Dutton, Schneider, Khosravi et al., Clinical characteristics and outcomes of critically ill patients with COVID-19 admitted to an intensive care unit in London: a prospective observational cohort study, PloS ONE, doi:10.1371/journal.pone.0243710
Yang, Ding, Xu, Pu, Li et al., Increased circulating level of interleukin-6 and CD8 + T cell exhaustion are associated with progression of COVID-19, Infect Dis Poverty, doi:10.1186/s40249-020-00780-6
Zumla, Hui, Azhar, Memish, Maeurer, Reducing mortality from 2019-nCoV: host-directed therapies should be an option, Lancet, doi:10.1016/S0140-6736(20)30305-6
DOI record:
{
"DOI": "10.1038/s41430-021-00984-5",
"ISSN": [
"0954-3007",
"1476-5640"
],
"URL": "http://dx.doi.org/10.1038/s41430-021-00984-5",
"alternative-id": [
"984"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "13 January 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Revised",
"name": "revised",
"order": 2,
"value": "1 July 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 3,
"value": "6 July 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 4,
"value": "23 July 2021"
},
{
"group": {
"label": "COMPETING INTERESTS",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "The authors declare no competing interests."
},
{
"label": "Free to read",
"name": "free",
"value": "This content has been made available to all."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-0752-8815",
"affiliation": [],
"authenticated-orcid": false,
"family": "Güven",
"given": "Mehmet",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-9394-4999",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gültekin",
"given": "Hamza",
"sequence": "additional"
}
],
"container-title": "European Journal of Clinical Nutrition",
"container-title-short": "Eur J Clin Nutr",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2021,
7,
23
]
],
"date-time": "2021-07-23T11:03:01Z",
"timestamp": 1627038181000
},
"deposited": {
"date-parts": [
[
2021,
9,
3
]
],
"date-time": "2021-09-03T12:08:51Z",
"timestamp": 1630670931000
},
"indexed": {
"date-parts": [
[
2024,
3,
13
]
],
"date-time": "2024-03-13T07:28:01Z",
"timestamp": 1710314881835
},
"is-referenced-by-count": 34,
"issue": "9",
"issued": {
"date-parts": [
[
2021,
7,
23
]
]
},
"journal-issue": {
"issue": "9",
"published-print": {
"date-parts": [
[
2021,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.springer.com/tdm",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
23
]
],
"date-time": "2021-07-23T00:00:00Z",
"timestamp": 1626998400000
}
},
{
"URL": "https://www.springer.com/tdm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
23
]
],
"date-time": "2021-07-23T00:00:00Z",
"timestamp": 1626998400000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41430-021-00984-5.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41430-021-00984-5",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41430-021-00984-5.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"page": "1383-1388",
"prefix": "10.1038",
"published": {
"date-parts": [
[
2021,
7,
23
]
]
},
"published-online": {
"date-parts": [
[
2021,
7,
23
]
]
},
"published-print": {
"date-parts": [
[
2021,
9
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1126/science.abf8003",
"doi-asserted-by": "publisher",
"key": "984_CR1",
"unstructured": "Pekar J, Worobey M, Moshiri N, Scheffler K, Wertheim JO. Timing the SARS-CoV-2 index case in Hubei province. Science. 2021;eabf8003. https://doi.org/10.1126/science.abf8003."
},
{
"key": "984_CR2",
"unstructured": "World Health Organization. Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020. http://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020. Accessed 12 Feb 2020."
},
{
"DOI": "10.1186/s40249-020-00780-6",
"author": "PH Yang",
"doi-asserted-by": "publisher",
"journal-title": "Infect Dis Poverty",
"key": "984_CR3",
"unstructured": "Yang PH, Ding YB, Xu Z, Pu R, Li P, Yan J, et al. Increased circulating level of interleukin-6 and CD8+ T cell exhaustion are associated with progression of COVID-19. Infect Dis Poverty 2020;9:161. https://doi.org/10.1186/s40249-020-00780-6.",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30305-6",
"author": "A Zumla",
"doi-asserted-by": "publisher",
"first-page": "e35",
"journal-title": "Lancet.",
"key": "984_CR4",
"unstructured": "Zumla A, Hui DS, Azhar EI, Memish ZA, Maeurer M. Reducing mortality from 2019-nCoV: host-directed therapies should be an option. Lancet. 2020;395:e35–6. https://doi.org/10.1016/S0140-6736(20)30305-6.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1530/EJE-20-0665",
"author": "JP Bilezikian",
"doi-asserted-by": "publisher",
"first-page": "R133",
"journal-title": "Eur J Endocrinol",
"key": "984_CR5",
"unstructured": "Bilezikian JP, Bikle D, Hewison M, Castro ML, Formenti AM, Gupta A, et al. MECHANISMS IN ENDOCRINOLOGY: vitamin D and COVID-19. Eur J Endocrinol. 2020;183:R133–47. https://doi.org/10.1530/EJE-20-0665.",
"volume": "183",
"year": "2020"
},
{
"DOI": "10.3390/ijerph18020740",
"author": "J Abraham",
"doi-asserted-by": "publisher",
"first-page": "740.",
"journal-title": "Int J Environ Res Public Health",
"key": "984_CR6",
"unstructured": "Abraham J, Dowling K, Florentine S. Can optimum solar radiation exposure or supplemented vitamin D intake reduce the severity of COVID-19 symptoms? Int J Environ Res Public Health. 2021;18:740. https://doi.org/10.3390/ijerph18020740.",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.3390/nu12072097",
"author": "N Charoenngam",
"doi-asserted-by": "publisher",
"first-page": "2097",
"journal-title": "Nutrients",
"key": "984_CR7",
"unstructured": "Charoenngam N, Holick MF. Immunologic effects of vitamin D on human health and disease. Nutrients. 2020;12:2097. https://doi.org/10.3390/nu12072097.",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.3390/nu12040988",
"author": "WB Grant",
"doi-asserted-by": "publisher",
"first-page": "988.",
"journal-title": "Nutrients.",
"key": "984_CR8",
"unstructured": "Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12:988. https://doi.org/10.3390/nu12040988.",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.3390/nu5072502",
"author": "B Prietl",
"doi-asserted-by": "publisher",
"first-page": "2502",
"journal-title": "Nutrients",
"key": "984_CR9",
"unstructured": "Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013;5:2502–21. https://doi.org/10.3390/nu5072502.",
"volume": "5",
"year": "2013"
},
{
"DOI": "10.4274/Tjem.1938",
"author": "G Tamer",
"doi-asserted-by": "publisher",
"first-page": "5",
"journal-title": "Turkjem",
"key": "984_CR10",
"unstructured": "Tamer G, Mesçi B. Role of vitamin D in the immune system. Turkjem. 2013;17:5–7. https://doi.org/10.4274/Tjem.1938.",
"volume": "17",
"year": "2013"
},
{
"DOI": "10.1016/j.jsbmb.2018.11.013",
"author": "CL Greiller",
"doi-asserted-by": "publisher",
"first-page": "152",
"journal-title": "J Steroid Biochem Mol Biol",
"key": "984_CR11",
"unstructured": "Greiller CL, Suri R, Jolliffe DA, Kebadze T, Hirsman AG, Griffiths CJ, et al. Vitamin D attenuates rhinovirus-induced expression of intercellular adhesion molecule-1 (ICAM-1) and platelet-activating factor receptor (PAFR) in respiratory epithelial cells. J Steroid Biochem Mol Biol. 2019;187:152–9. https://doi.org/10.1016/j.jsbmb.2018.11.013.",
"volume": "187",
"year": "2019"
},
{
"DOI": "10.1371/journal.pone.0086755",
"author": "RA Brockman-Schneider",
"doi-asserted-by": "publisher",
"first-page": "e86755",
"journal-title": "PLoS ONE",
"key": "984_CR12",
"unstructured": "Brockman-Schneider RA, Pickles RJ, Gern JE. Effects of vitamin D on airway epithelial cell morphology and rhinovirus replication. PLoS ONE. 2014;9:e86755. https://doi.org/10.1371/journal.pone.0086755.",
"volume": "9",
"year": "2014"
},
{
"DOI": "10.1074/jbc.M113.467670",
"author": "Y Chen",
"doi-asserted-by": "publisher",
"first-page": "19450",
"journal-title": "J Biol Chem",
"key": "984_CR13",
"unstructured": "Chen Y, Zhang J, Ge X, Du J, Deb DK, Li YC. Vitamin D receptor inhibits nuclear factor κ B activation by interacting with IκB kinase β protein. J Biol Chem. 2013;288:19450–8. https://doi.org/10.1074/jbc.M113.467670.",
"volume": "288",
"year": "2013"
},
{
"DOI": "10.1136/bmj.i6583",
"author": "AR Martineau",
"doi-asserted-by": "publisher",
"first-page": "i6583",
"journal-title": "BMJ.",
"key": "984_CR14",
"unstructured": "Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. https://doi.org/10.1136/bmj.i6583.",
"volume": "356",
"year": "2017"
},
{
"DOI": "10.1096/fj.04-3284com",
"author": "AF Gombart",
"doi-asserted-by": "publisher",
"first-page": "1067",
"journal-title": "FASEB J",
"key": "984_CR15",
"unstructured": "Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–77. https://doi.org/10.1096/fj.04-3284com.",
"volume": "19",
"year": "2005"
},
{
"DOI": "10.1111/j.1365-2249.2009.04001.x",
"author": "DA Hughes",
"doi-asserted-by": "publisher",
"first-page": "20",
"journal-title": "Clin Exp Immunol",
"key": "984_CR16",
"unstructured": "Hughes DA, Norton R. Vitamin D and respiratory health. Clin Exp Immunol. 2009;158:20–5. https://doi.org/10.1111/j.1365-2249.2009.04001.x.",
"volume": "158",
"year": "2009"
},
{
"DOI": "10.1038/s41430-020-0661-0",
"author": "M Ebadi",
"doi-asserted-by": "publisher",
"first-page": "856",
"journal-title": "Eur J Clin Nutr",
"key": "984_CR17",
"unstructured": "Ebadi M, Montano-Loza AJ. Perspective: improving vitamin D status in the management of COVID-19. Eur J Clin Nutr. 2020;74:856–9. https://doi.org/10.1038/s41430-020-0661-0.",
"volume": "74",
"year": "2020"
},
{
"DOI": "10.23812/CONTI-E",
"doi-asserted-by": "publisher",
"key": "984_CR18",
"unstructured": "Conti P, Ronconi G, Caraffa A, Gallenga CE, Ross R, Frydas I, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34. https://doi.org/10.23812/CONTI-E."
},
{
"DOI": "10.1002/jmv.26360",
"author": "R Munshi",
"doi-asserted-by": "publisher",
"first-page": "733",
"journal-title": "J Med Virol",
"key": "984_CR19",
"unstructured": "Munshi R, Hussein MH, Toraih EA, Elshazli RM, Jardak C, Sultana N, et al. Vitamin D insufficiency as a potential culprit in critical COVID-19 patients. J Med Virol. 2021;93:733–40. https://doi.org/10.1002/jmv.26360.",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1111/febs.15495",
"author": "E Merzon",
"doi-asserted-by": "publisher",
"first-page": "3693",
"journal-title": "FEBS J",
"key": "984_CR20",
"unstructured": "Merzon E, Tworowski D, Gorohovski A, Vinker S, Cohen AG, Green I, et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J. 2020;287:3693–702. https://doi.org/10.1111/febs.15495.",
"volume": "287",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0239799",
"author": "Z Maghbooli",
"doi-asserted-by": "publisher",
"first-page": "e0239799.",
"journal-title": "PLoS ONE",
"key": "984_CR21",
"unstructured": "Maghbooli Z, Sahraian MA, Ebrahimi M, Pazoki M, Kafan S, Tabriz HM, et al. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS ONE. 2020;15:e0239799. https://doi.org/10.1371/journal.pone.0239799.",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1016/S2213-8587(20)30183-2",
"author": "F Mitchell",
"doi-asserted-by": "publisher",
"first-page": "570.",
"journal-title": "Lancet Diabetes Endocrinol",
"key": "984_CR22",
"unstructured": "Mitchell F. Vitamin-D and COVID-19: do deficient risk a poorer outcome? Lancet Diabetes Endocrinol. 2020;8:570. https://doi.org/10.1016/S2213-8587(20)30183-2.",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/S2213-8587(20)30268-0",
"author": "AR Martineau",
"doi-asserted-by": "publisher",
"first-page": "735",
"journal-title": "Lancet Diabetes Endocrinol",
"key": "984_CR23",
"unstructured": "Martineau AR, Forouhi NG. Vitamin D for COVID-19: a case to answer? Lancet Diabetes Endocrinol. 2020;8:735–6. https://doi.org/10.1016/S2213-8587(20)30268-0.",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0243710",
"author": "RJ Thomson",
"doi-asserted-by": "publisher",
"first-page": "e0243710",
"journal-title": "PloS ONE",
"key": "984_CR24",
"unstructured": "Thomson RJ, Hunter J, Dutton J, Schneider J, Khosravi M, Casement A, et al. Clinical characteristics and outcomes of critically ill patients with COVID-19 admitted to an intensive care unit in London: a prospective observational cohort study. PloS ONE. 2020;15:e0243710. https://doi.org/10.1371/journal.pone.0243710.",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1590/1516-3180.2020.0722.R1.2302021",
"doi-asserted-by": "publisher",
"key": "984_CR25",
"unstructured": "Güven M, Gültekin H. Could serum total cortisol level at admission predict mortality due to coronavirus disease 2019 in the intensive care unit? A prospective study. Sao Paulo Med J. 2021;S1516-31802021005016206. https://doi.org/10.1590/1516-3180.2020.0722.R1.2302021."
},
{
"DOI": "10.1111/ijcp.13803",
"doi-asserted-by": "publisher",
"key": "984_CR26",
"unstructured": "Solmaz I, Özçaylak S, Alakuş ÖF, Kılıç J, Kalın BS, Güven M, et al. Risk factors affecting ICU admission in COVID-19 patients; Could air temperature be an effective factor? Int J Clin Pract. 2020;e13803. https://doi.org/10.1111/ijcp.13803."
},
{
"DOI": "10.3906/sag-2007-97",
"doi-asserted-by": "publisher",
"key": "984_CR27",
"unstructured": "Asan A, Üstündağ Y, Koca N, Şimşek A, Sayan HE, Parildar H, et al. Do initial hematologic indices predict the severity of COVID-19 patients? Turk J Med Sci. 2020;10.3906/sag-2007-97. https://doi.org/10.3906/sag-2007-97."
},
{
"DOI": "10.1183/13993003.00547-2020",
"author": "WJ Guan",
"doi-asserted-by": "publisher",
"first-page": "2000547.",
"journal-title": "Eur Respir J",
"key": "984_CR28",
"unstructured": "Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. https://doi.org/10.1183/13993003.00547-2020.",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1093/gerona/glaa183",
"author": "JL Atkins",
"doi-asserted-by": "publisher",
"first-page": "2224",
"journal-title": "J Gerontol A Biol Sci Med Sci",
"key": "984_CR29",
"unstructured": "Atkins JL, Masoli JAH, Delgado J, Pilling LC, Kuo CL, Kuchel GA, et al. Preexisting comorbidities predicting COVID-19 and mortality in the UK Biobank Community Cohort. J Gerontol A Biol Sci Med Sci. 2020;75:2224–30. https://doi.org/10.1093/gerona/glaa183.",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1016/j.clnesp.2020.10.008",
"author": "TJM Gonçalves",
"doi-asserted-by": "publisher",
"first-page": "110",
"journal-title": "Clin Nutr ESPEN",
"key": "984_CR30",
"unstructured": "Gonçalves TJM, Gonçalves SEAB, Guarnieri A, Risegato RC, Guimarães MP, Freitas DCD, et al. Prevalence of obesity and hypovitaminosis D in elderly with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Nutr ESPEN. 2020;40:110–4. https://doi.org/10.1016/j.clnesp.2020.10.008.",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2020.19722",
"author": "DO Meltzer",
"doi-asserted-by": "publisher",
"first-page": "e2019722.",
"journal-title": "JAMA Netw Open",
"key": "984_CR31",
"unstructured": "Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw Open. 2020;3:e2019722. https://doi.org/10.1001/jamanetworkopen.2020.19722.",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0239252",
"author": "HW Kaufman",
"doi-asserted-by": "publisher",
"first-page": "e0239252.",
"journal-title": "PLoS ONE",
"key": "984_CR32",
"unstructured": "Kaufman HW, Niles JK, Kroll MH, Bi C, Holick MF. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS ONE. 2020;15:e0239252. https://doi.org/10.1371/journal.pone.0239252.",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.26848",
"author": "IH Murai",
"doi-asserted-by": "publisher",
"first-page": "1053",
"journal-title": "JAMA",
"key": "984_CR33",
"unstructured": "Murai IH, Fernandes AL, Sales LP, Pinto AJ, Goessler KF, Duran CSC, et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA. 2021;325:1053–60. https://doi.org/10.1001/jama.2020.26848.",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1016/j.jcte.2016.04.004",
"author": "JE Han",
"doi-asserted-by": "publisher",
"first-page": "59",
"journal-title": "J Clin Transl Endocrinol",
"key": "984_CR34",
"unstructured": "Han JE, Jones JL, Tangpricha V, Brown MA, Brown LAS, Hao L, et al. High dose vitamin D administration in ventilated intensive care unit patients: a pilot double blind randomized controlled trial. J Clin Transl Endocrinol. 2016;4:59–65. https://doi.org/10.1016/j.jcte.2016.04.004.",
"volume": "4",
"year": "2016"
},
{
"DOI": "10.1001/jama.2014.13204",
"author": "K Amrein",
"doi-asserted-by": "publisher",
"first-page": "1520",
"journal-title": "JAMA",
"key": "984_CR35",
"unstructured": "Amrein K, Schnedl C, Holl A, Riedl R, Christopher KB, Pachler C, et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA. 2014;312:1520–30. https://doi.org/10.1001/jama.2014.13204.",
"volume": "312",
"year": "2014"
},
{
"DOI": "10.1007/s00394-020-02372-4",
"doi-asserted-by": "publisher",
"key": "984_CR36",
"unstructured": "Hastie CE, Pell JP, Sattar N. Vitamin D and COVID-19 infection and mortality in UK Biobank. Eur J Nutr. 2020;1–4. https://doi.org/10.1007/s00394-020-02372-4."
},
{
"DOI": "10.1136/bmjnph-2020-000085",
"author": "PC Calder",
"doi-asserted-by": "publisher",
"first-page": "74",
"journal-title": "BMJ Nutr Prev Health",
"key": "984_CR37",
"unstructured": "Calder PC. Nutrition, immunity and COVID-19. BMJ Nutr Prev Health. 2020;3:74–92. https://doi.org/10.1136/bmjnph-2020-000085.",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1016/j.jsbmb.2020.105751",
"author": "M Entrenas Castillo",
"doi-asserted-by": "publisher",
"first-page": "105751.",
"journal-title": "J Steroid Biochem Mol Biol.",
"key": "984_CR38",
"unstructured": "Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, Alcalá Díaz JF, Miranda JL, Bouillon R, et al. “Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study”. J Steroid Biochem Mol Biol. 2020;203:105751. https://doi.org/10.1016/j.jsbmb.2020.105751.",
"volume": "203",
"year": "2020"
}
],
"reference-count": 38,
"references-count": 38,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41430-021-00984-5"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Nutrition and Dietetics",
"Medicine (miscellaneous)"
],
"subtitle": [],
"title": "The effect of high-dose parenteral vitamin D3 on COVID-19-related inhospital mortality in critical COVID-19 patients during intensive care unit admission: an observational cohort study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "75"
}
