Real-world efficacy and safety of azvudine in hospitalized older patients with COVID-19 during the omicron wave in China: A retrospective cohort study
et al., Acta Pharmaceutica Sinica B, doi:10.1016/j.apsb.2024.12.004, ChiCTR2300072750, Dec 2024
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.0000000041 from 40 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
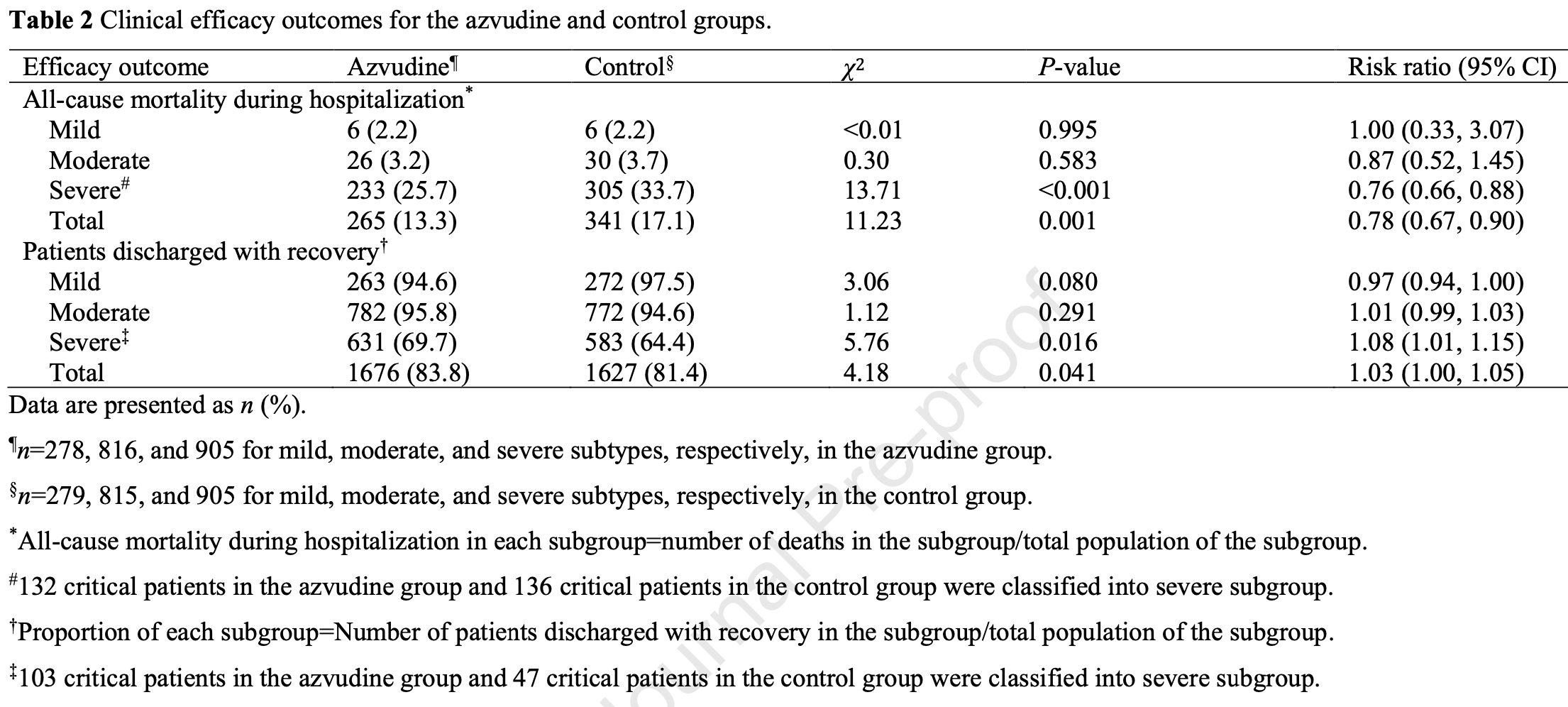
PSM retrospective 3,998 hospitalized COVID-19 patients aged 60 years and older in China showing lower all-cause mortality, higher rate of discharge, and shorter time to viral clearance with azvudine treatment.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments3.
|
risk of death, 22.0% lower, RR 0.78, p = 0.001, treatment 265 of 1,999 (13.3%), control 341 of 1,999 (17.1%), NNT 26, propensity score matching.
|
|
risk of no hospital discharge, 13.2% lower, RR 0.87, p = 0.045, treatment 323 of 1,999 (16.2%), control 372 of 1,999 (18.6%), NNT 41, propensity score matching.
|
|
hospitalization time, 1.4% lower, relative time 0.99, p = 0.43, treatment mean 13.8 (±6.2) n=1,676, control mean 14.0 (±8.2) n=1,623.
|
|
time to viral-, 10.4% lower, relative time 0.90, p < 0.001, treatment mean 12.9 (±6.6) n=1,676, control mean 14.4 (±9.5) n=1,623.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Xiong et al., Real-world data of Azvudine-induced hepatotoxicity among hospitalized COVID-19 patients in China: a retrospective case-control study, Frontiers in Pharmacology, doi:10.3389/fphar.2025.1558054.
Zhu et al., 12 Dec 2024, retrospective, China, peer-reviewed, 55 authors, study period 1 December, 2022 - 28 February, 2023, trial ChiCTR2300072750.
Contact: j790101@163.com.
Real-world efficacy and safety of azvudine in hospitalized older patients with COVID-19 during the omicron wave in China: A retrospective cohort study
Acta Pharmaceutica Sinica B, doi:10.1016/j.apsb.2024.12.004
This multicenter, retrospective cohort study indicates that azvudine can reduce the all-cause mortality rate and shorten the time to nucleic acid-negative conversion in the elderly Chinese patients hospitalized due to COVID-19 infection.
Author contributions Yuanchao Zhu, Fei Zhao, and Pengfei Jin designed the study. Pengfei Jin led the study team. The underlying data at each subcenter were acquired by Xingang Fei Zhao and Yubing Zhu analysed the data. Yubing Zhu and Fei Zhao wrote the first draft of the manuscript, which was revised by Pengfei Jin and Yuanchao Zhu. All authors interpreted data, provided critical review and revision of the text, and approved the final version of the manuscript. All authors had access to the data underlying the study and accept responsibility for the decision to submit for publication.
Conflicts of interest All researchers can confirm their independence from funders, and all authors, external and internal, had full access to all the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
National Health Commission Clinical Research Projects for Innovative Drugs and National High Level Hospital Clinical Research Funding had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or J o u r n a l P r e -p r o o f
References
Austin, Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations, Biom J
Cao, Gao, Bao, Feng, Mei et al., VV116 versus nirmatrelvir-ritonavir for oral treatment of Covid-19, N Engl J Med
Center, Control, Epidemic situation of novel coronavirus infection in China
Chen, Klein, Garibaldi, Li, Wu et al., Aging in COVID-19: vulnerability, immunity and intervention, Ageing Res Rev
Chen, Tian, Efficacy and safety of azvudine in patients with COVID-19: a systematic review and meta-analysis, Heliyon
Chen, Xu, Hong, Yang, Peng et al., Oral azvudine (FNC) tablets in patients infected with SARS-CoV-2 omicron variant: a retrospective cohort study, medRxiv
Cui, Liu, Wang, Wang, Fan et al., Structural and functional characterizations of infectivity and immune evasion of SARS-CoV-2 Omicron, Cell
Da Silva, Abreu Cabral, De Souza, Arruda, Cabral et al., Serial viral load analysis by DDPCR to evaluate FNC efficacy and safety in the treatment of mild cases of COVID-19, Front Med
De Souza, Cabral, Da Silva, Arruda, Cabral et al., Phase III, randomized, double-blind, placebo-controlled clinical study: a study on the safety and clinical efficacy of AZVUDINE in moderate COVID-19 patients, Front Med
Deng, Li, Sun, Zhou, Xiao, Real-world effectiveness of azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: a retrospective cohort study, J Med Virol
J O U R N A L P R E, None
J O U R N A L P R E, None
Li, Hilgenfeld, Whitley, Clercq, Therapeutic strategies for COVID-19: progress and lessons learned, Nat Rev Drug Discov
Shang, Fu, Geng, Zhang, Zhang et al., Azvudine therapy of common COVID-19 in hemodialysis patients, J Med Virol
Smith, Kalayanov, Sund, Winqvist, Maltseva et al., The design, synthesis, and antiviral activity of monofluoro and difluoro analogues of 4′-azidocytidine against hepatitis C virus replication: the discovery of 4′-azido-2′-deoxy-2′-fluorocytidine and 4′-azido-2′dideoxy-2′,2′-difluorocytidine, J Med Chem
Sun, Dian, Shen, Zeng, Chen, Oral azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study, EClinicalMedicine
Sun, Peng, Yu, Zhang, Liang et al., Mechanistic insight into antiretroviral potency of 2′-Deoxy-2′-β-fluoro-4′-azidocytidine (FNC) with a long-lasting effect on HIV-1 prevention, J Med Chem
Wang, Pan, Zhang, Han, Fan et al., Epidemiological and clinical features of 125 hospitalized patients with COVID-19 in Fuyang, Anhui, China, Int J Infect Dis
Wang, Sun, Zhang, Li, Qin et al., Antiviral effectiveness and survival correlation of azvudine and nirmatrelvir/ritonavir in elderly severe patients with COVID-19: a retrospective real-world study, EClinicalMedicine
Wang, Yao, Wang, Gong, Meng et al., Determinants of COVID-19 vaccination status and hesitancy among older adults in China, Nat Med
Wei, Zeng, Wang, Gui, Zhang et al., Head-to-head comparison of azvudine and nirmatrelvir/ritonavir for the hospitalized patients with COVID-19: a real-world retrospective cohort study with propensity score matching, Front Pharmacol
Yang, Wang, Jiang, Zhang, Zhang et al., Oral azvudine for mild-tomoderate COVID-19 in high risk, nonhospitalized adults: results of a real-world study, J Med Virol
Yu, Chang, Azvudine (FNC): a promising clinical candidate for COVID-19 treatment, Signal Transduct Target Ther
Yu, Chang, The first Chinese oral anti-COVID-19 drug Azvudine launched, Innovation (Camb)
Zhang, Li, Wang, Liu, Lu et al., Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients, Signal Transduct Target Ther
Zhao, Zheng, Han, Feng, Xia et al., Is azvudine comparable to nirmatrelvir-ritonavir in real-world efficacy and safety for hospitalized patients with COVID-19? A retrospective cohort study, Infect Dis Ther
Zheng, Wang, Yang, Guo, Chen et al., Antiviral activity of FNC, 2′-deoxy-2′-β-fluoro-4′-azidocytidine, against human and duck HBV replication, Antivir Ther
DOI record:
{
"DOI": "10.1016/j.apsb.2024.12.004",
"ISSN": [
"2211-3835"
],
"URL": "http://dx.doi.org/10.1016/j.apsb.2024.12.004",
"alternative-id": [
"S2211383524004611"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Real-world efficacy and safety of azvudine in hospitalized older patients with COVID-19 during the omicron wave in China: A retrospective cohort study"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Acta Pharmaceutica Sinica B"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.apsb.2024.12.004"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 The Authors. Published by Elsevier B.V. on behalf of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences."
}
],
"author": [
{
"affiliation": [],
"family": "Zhu",
"given": "Yuanchao",
"sequence": "first"
},
{
"affiliation": [],
"family": "Zhao",
"given": "Fei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhu",
"given": "Yubing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Xingang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dong",
"given": "Deshi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhu",
"given": "Bolin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Jianchun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hu",
"given": "Xin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhao",
"given": "Zinan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xu",
"given": "Wenfeng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jv",
"given": "Yang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Dandan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zheng",
"given": "Yingming",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dong",
"given": "Yiwen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Lu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Shilei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Teng",
"given": "Zhiyuan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lu",
"given": "Ling",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhu",
"given": "Jingwei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Du",
"given": "Linzhe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Yunxin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jia",
"given": "Lechuan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Qiujv",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ma",
"given": "Hui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhao",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jiang",
"given": "Hongliu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xu",
"given": "Xin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Jinli",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Qian",
"given": "Xuping",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Wei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zheng",
"given": "Tingting",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Chunxia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Xuguang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Kun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jiang",
"given": "Huanhuan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Qu",
"given": "Dongxiang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Song",
"given": "Jia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cheng",
"given": "Hua",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sun",
"given": "Wenfang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhan",
"given": "Hanqiu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Xiao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Yafeng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Aixia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Li",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Lihua",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Nan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Shumin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ma",
"given": "Jingjing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Wei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Du",
"given": "Xiaoxiang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zheng",
"given": "Meiqin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wan",
"given": "Liyan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Du",
"given": "Guangqing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Hangmei",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-9461-0453",
"affiliation": [],
"authenticated-orcid": false,
"family": "Jin",
"given": "Pengfei",
"sequence": "additional"
}
],
"container-title": "Acta Pharmaceutica Sinica B",
"container-title-short": "Acta Pharmaceutica Sinica B",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
12,
12
]
],
"date-time": "2024-12-12T07:09:16Z",
"timestamp": 1733987356000
},
"deposited": {
"date-parts": [
[
2024,
12,
12
]
],
"date-time": "2024-12-12T17:31:38Z",
"timestamp": 1734024698000
},
"indexed": {
"date-parts": [
[
2024,
12,
13
]
],
"date-time": "2024-12-13T05:28:32Z",
"timestamp": 1734067712303,
"version": "3.30.2"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
12
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
12,
1
]
],
"date-time": "2024-12-01T00:00:00Z",
"timestamp": 1733011200000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
12,
1
]
],
"date-time": "2024-12-01T00:00:00Z",
"timestamp": 1733011200000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 3,
"start": {
"date-parts": [
[
2024,
12,
4
]
],
"date-time": "2024-12-04T00:00:00Z",
"timestamp": 1733270400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2211383524004611?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2211383524004611?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
12
]
]
},
"published-print": {
"date-parts": [
[
2024,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.apsb.2024.12.004_bib1",
"unstructured": "Chinese Center for Disease Control and Prevention. Epidemic situation of novel coronavirus infection in China. [2023-4-1] [2024-3-24]. Available from: https://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_13141/202304/t20230401_264798.html."
},
{
"DOI": "10.1016/j.cell.2022.01.019",
"article-title": "Structural and functional characterizations of infectivity and immune evasion of SARS-CoV-2 Omicron",
"author": "Cui",
"doi-asserted-by": "crossref",
"first-page": "860",
"journal-title": "Cell",
"key": "10.1016/j.apsb.2024.12.004_bib2",
"volume": "185",
"year": "2022"
},
{
"key": "10.1016/j.apsb.2024.12.004_bib3",
"unstructured": "World Health Organization. WHO COVID-19 dashboard. [2023-2-31] [2024-3-24]. Available from: https://data.who.int/dashboards/covid19/cases?m49=156&n=c."
},
{
"article-title": "The first Chinese oral anti-COVID-19 drug Azvudine launched",
"author": "Yu",
"journal-title": "Innovation (Camb)",
"key": "10.1016/j.apsb.2024.12.004_bib4",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1021/acs.jmedchem.0c00940",
"article-title": "Mechanistic insight into antiretroviral potency of 2′-Deoxy-2′-β-fluoro-4′-azidocytidine (FNC) with a long-lasting effect on HIV-1 prevention",
"author": "Sun",
"doi-asserted-by": "crossref",
"first-page": "8554",
"journal-title": "J Med Chem",
"key": "10.1016/j.apsb.2024.12.004_bib5",
"volume": "63",
"year": "2020"
},
{
"article-title": "Serial viral load analysis by DDPCR to evaluate FNC efficacy and safety in the treatment of mild cases of COVID-19",
"author": "da Silva",
"journal-title": "Front Med (Lausanne)",
"key": "10.1016/j.apsb.2024.12.004_bib6",
"volume": "10",
"year": "2023"
},
{
"article-title": "Panel of expert consensus on the application of azvudine tablets in the treatment of SARS-COV-2 infection. Expert consensus on the application of azvudine tablets in the treatment of SARS-COV-2 infection",
"first-page": "1",
"journal-title": "China Pharm",
"key": "10.1016/j.apsb.2024.12.004_bib7",
"volume": "32",
"year": "2023"
},
{
"article-title": "Phase III, randomized, double-blind, placebo-controlled clinical study: a study on the safety and clinical efficacy of AZVUDINE in moderate COVID-19 patients",
"author": "de Souza",
"journal-title": "Front Med (Lausanne)",
"key": "10.1016/j.apsb.2024.12.004_bib8",
"volume": "10",
"year": "2023"
},
{
"key": "10.1016/j.apsb.2024.12.004_bib9",
"unstructured": "National Medical Products Administration. NMPA conditionally approved Henan Real Biotechnology Co., Ltd. Azfudine tablets to add the novel coronavirus treatment indication registration application. [2022-7-25] [2024-3-24]. Available from: https://www.nmpa.gov.cn/yaowen/ypjgyw/ypyw/20220725165620176.html."
},
{
"key": "10.1016/j.apsb.2024.12.004_bib10",
"unstructured": "Medical Administration Bureau of the National Health Commission. Notice on including azvudine tablets into the diagnosis and treatment guideine of novel coronavirus pneumonia. [2022-8-9] [2024-3-24]. Available from: http://www.nhc.gov.cn/yzygj/s7653p/202208/33e3ff4308b4446796c3f315601d436f.shtml."
},
{
"DOI": "10.1016/j.eclinm.2023.101981",
"article-title": "Oral azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study",
"author": "Sun",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "10.1016/j.apsb.2024.12.004_bib11",
"volume": "59",
"year": "2023"
},
{
"DOI": "10.1002/jmv.28756",
"article-title": "Real-world effectiveness of azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: a retrospective cohort study",
"author": "Deng",
"doi-asserted-by": "crossref",
"journal-title": "J Med Virol",
"key": "10.1016/j.apsb.2024.12.004_bib12",
"volume": "95",
"year": "2023"
},
{
"article-title": "Efficacy and safety of azvudine in patients with COVID-19: a systematic review and meta-analysis",
"author": "Chen",
"journal-title": "Heliyon",
"key": "10.1016/j.apsb.2024.12.004_bib13",
"volume": "9",
"year": "2023"
},
{
"DOI": "10.1002/jmv.28947",
"article-title": "Oral azvudine for mild-to-moderate COVID-19 in high risk, nonhospitalized adults: results of a real-world study",
"author": "Yang",
"doi-asserted-by": "crossref",
"journal-title": "J Med Virol",
"key": "10.1016/j.apsb.2024.12.004_bib14",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1016/j.arr.2020.101205",
"article-title": "Aging in COVID-19: vulnerability, immunity and intervention",
"author": "Chen",
"doi-asserted-by": "crossref",
"journal-title": "Ageing Res Rev",
"key": "10.1016/j.apsb.2024.12.004_bib15",
"volume": "65",
"year": "2021"
},
{
"key": "10.1016/j.apsb.2024.12.004_bib16",
"unstructured": "World Health Organization. COVID-19 cases and deaths with age and sex reported. [2023-8-28] [2024-3-24]. Available from: https://app.powerbi.com/view?r=eyJrIjoiY2UyNmQ0MWQtYjdiZC00MmIyLWI5YmYtZmRiZWJkZDcyMDMwIiwidCI6ImY2MTBjMGI3LWJkMjQtNGIzOS04MTBiLTNkYzI4MGFmYjU5MCIsImMiOjh9."
},
{
"DOI": "10.1016/j.ijid.2020.03.070",
"article-title": "Epidemiological and clinical features of 125 hospitalized patients with COVID-19 in Fuyang, Anhui, China",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "421",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.apsb.2024.12.004_bib17",
"volume": "95",
"year": "2020"
},
{
"key": "10.1016/j.apsb.2024.12.004_bib18",
"unstructured": "General Office of the National Health Commission. Notice on the issuance of diagnosis and treatment protocol for novel coronavirus infection (trial version 10). [2023-1-5] [2024-3-24]. Available from: https://www.gov.cn/zhengce/zhengceku/2023-01/06/content_5735343.htm."
},
{
"DOI": "10.1056/NEJMoa2208822",
"article-title": "VV116 versus nirmatrelvir–ritonavir for oral treatment of Covid-19",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "406",
"journal-title": "N Engl J Med",
"key": "10.1016/j.apsb.2024.12.004_bib19",
"volume": "388",
"year": "2023"
},
{
"key": "10.1016/j.apsb.2024.12.004_bib20",
"unstructured": "National Institutes of Health. Common terminology criteria for adverse events. [2021-4-19] [2024-3-24]. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm."
},
{
"article-title": "Oral azvudine (FNC) tablets in patients infected with SARS-CoV-2 omicron variant: a retrospective cohort study",
"author": "Chen",
"journal-title": "medRxiv",
"key": "10.1016/j.apsb.2024.12.004_bib21",
"year": "2023"
},
{
"DOI": "10.1002/bimj.200810488",
"article-title": "Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations",
"author": "Austin",
"doi-asserted-by": "crossref",
"first-page": "171",
"journal-title": "Biom J",
"key": "10.1016/j.apsb.2024.12.004_bib22",
"volume": "51",
"year": "2009"
},
{
"DOI": "10.1038/s41573-023-00672-y",
"article-title": "Therapeutic strategies for COVID-19: progress and lessons learned",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "449",
"journal-title": "Nat Rev Drug Discov",
"key": "10.1016/j.apsb.2024.12.004_bib23",
"volume": "22",
"year": "2023"
},
{
"DOI": "10.1038/s41392-021-00835-6",
"article-title": "Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "414",
"journal-title": "Signal Transduct Target Ther",
"key": "10.1016/j.apsb.2024.12.004_bib24",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.3851/IMP2094",
"article-title": "Antiviral activity of FNC, 2′-deoxy-2′-β-fluoro-4′-azidocytidine, against human and duck HBV replication",
"author": "Zheng",
"doi-asserted-by": "crossref",
"first-page": "679",
"journal-title": "Antivir Ther",
"key": "10.1016/j.apsb.2024.12.004_bib25",
"volume": "17",
"year": "2012"
},
{
"DOI": "10.1021/jm801595c",
"article-title": "The design, synthesis, and antiviral activity of monofluoro and difluoro analogues of 4′-azidocytidine against hepatitis C virus replication: the discovery of 4′-azido-2′-deoxy-2′-fluorocytidine and 4′-azido-2′-dideoxy-2′,2′-difluorocytidine",
"author": "Smith",
"doi-asserted-by": "crossref",
"first-page": "2971",
"journal-title": "J Med Chem",
"key": "10.1016/j.apsb.2024.12.004_bib26",
"volume": "52",
"year": "2009"
},
{
"DOI": "10.1038/s41392-020-00351-z",
"article-title": "Azvudine (FNC): a promising clinical candidate for COVID-19 treatment",
"author": "Yu",
"doi-asserted-by": "crossref",
"first-page": "236",
"journal-title": "Signal Transduct Target Ther",
"key": "10.1016/j.apsb.2024.12.004_bib27",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1007/s40121-023-00845-7",
"article-title": "Is azvudine comparable to nirmatrelvir–ritonavir in real-world efficacy and safety for hospitalized patients with COVID-19? A retrospective cohort study",
"author": "Zhao",
"doi-asserted-by": "crossref",
"first-page": "2087",
"journal-title": "Infect Dis Ther",
"key": "10.1016/j.apsb.2024.12.004_bib28",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.3389/fphar.2023.1274294",
"article-title": "Head-to-head comparison of azvudine and nirmatrelvir/ritonavir for the hospitalized patients with COVID-19: a real-world retrospective cohort study with propensity score matching",
"author": "Wei",
"doi-asserted-by": "crossref",
"journal-title": "Front Pharmacol",
"key": "10.1016/j.apsb.2024.12.004_bib29",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1016/j.eclinm.2024.102468",
"article-title": "Antiviral effectiveness and survival correlation of azvudine and nirmatrelvir/ritonavir in elderly severe patients with COVID-19: a retrospective real-world study",
"author": "Wang",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "10.1016/j.apsb.2024.12.004_bib30",
"volume": "69",
"year": "2024"
},
{
"DOI": "10.1002/jmv.29007",
"article-title": "Azvudine therapy of common COVID-19 in hemodialysis patients",
"author": "Shang",
"doi-asserted-by": "crossref",
"journal-title": "J Med Virol",
"key": "10.1016/j.apsb.2024.12.004_bib31",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1038/s41591-023-02241-7",
"article-title": "Determinants of COVID-19 vaccination status and hesitancy among older adults in China",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "623",
"journal-title": "Nat Med",
"key": "10.1016/j.apsb.2024.12.004_bib32",
"volume": "29",
"year": "2023"
}
],
"reference-count": 32,
"references-count": 32,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2211383524004611"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Real-world efficacy and safety of azvudine in hospitalized older patients with COVID-19 during the omicron wave in China: A retrospective cohort study",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy"
}
