The effectiveness and safety of azvudine treatment in COVID‐19 patients with kidney disease based on a multicenter retrospective cohort study
et al., VIEW, doi:10.1002/VIW.20240075, NCT06349655, Apr 2025
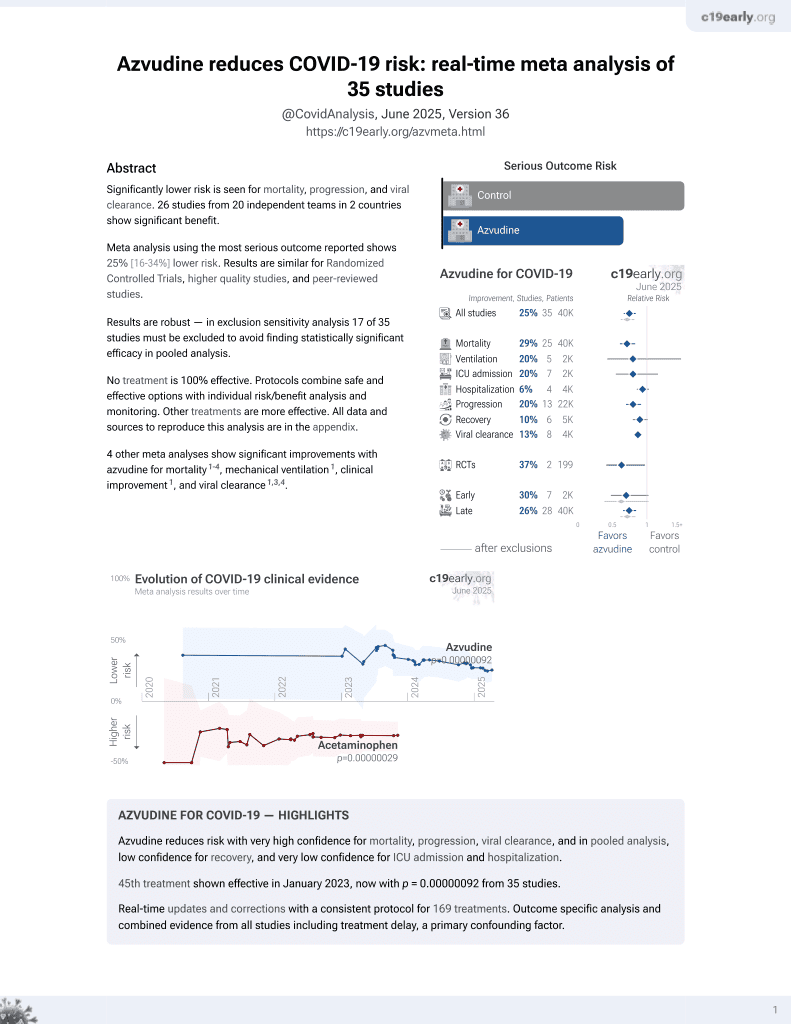
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.000000017 from 39 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
PSM retrospective 4,192 hospitalized COVID-19 patients with kidney disease showing significantly reduced all-cause mortality and disease progression with azvudine.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments3.
|
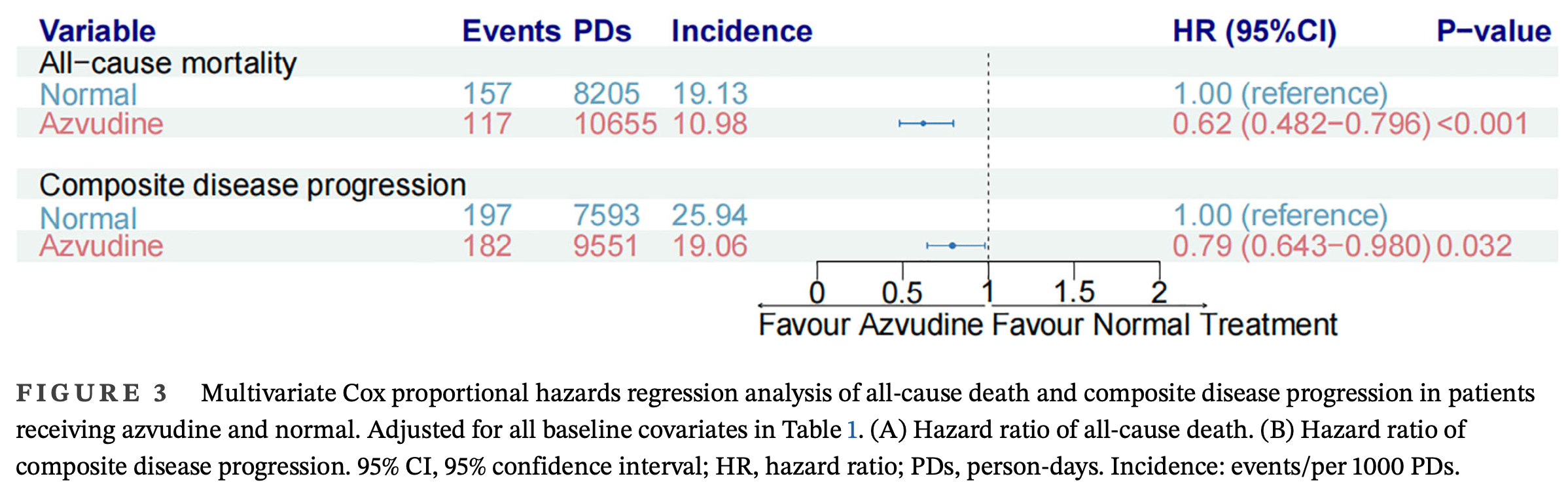
risk of death, 38.0% lower, HR 0.62, p < 0.001, treatment 831, control 831, adjusted per study, propensity score matching, multivariable, Cox proportional hazards.
|
|
risk of progression, 21.0% lower, HR 0.79, p = 0.03, treatment 831, control 831, adjusted per study, propensity score matching, multivariable, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Xiong et al., Real-world data of Azvudine-induced hepatotoxicity among hospitalized COVID-19 patients in China: a retrospective case-control study, Frontiers in Pharmacology, doi:10.3389/fphar.2025.1558054.
Yu et al., 9 Apr 2025, retrospective, China, peer-reviewed, mean age 64.4, 14 authors, study period 5 December, 2022 - 31 January, 2023, trial NCT06349655 (history).
Contact: fccrenzg@zzu.edu.cn, kanqc@zzu.edu.cn.
The effectiveness and safety of azvudine treatment in COVID‐19 patients with kidney disease based on a multicenter retrospective cohort study
VIEW, doi:10.1002/viw.20240075
Kidney disease has been the main risk factor of poor prognosis for COVID-19 patients. The effectiveness and safety of azvudine treatment in COVID-19 patients with kidney disease have not been reported. Herein, we conducted a nine-center and retrospective cohort study in China (ClinicalTrials: NCT06349655) that enrolled 32,864 hospitalized COVID-19 patients, in which 4192 patients were pre-existed with kidney disease. After exclusions and propensity score matching, a total of 831 kidney disease patients treated with azvudine and 831 kidney disease patients treated without any anti-viral treatment (normal group) were selected. Based on Kaplan-Meier and Cox regression analysis, we found that azvudine administration had significantly decreased risks of all-cause death (p < 0.0001) and composite disease progression (p = 0.012) as compared Bo Yu, Mengzhao Yang, and Jia Yu contributed equally to this work.
S U P P O R T I N G I N F O R M AT I O N Additional supporting information can be found online in the Supporting Information section at the end of this article.
References
Bellocco, Pagano, None, Nutrition
Cai, Zhang, Zhu, Liu, Liu et al., None, Int. Urol. Nephrol
Cheng, Luo, Wang, Zhang, Wang et al., None, Kidney Int
China, COVID-19 diagnosis and treatment plan (trial version 10)
China, COVID-19 diagnosis and treatment plan (trial version 9)
Deng, Li, Sun, Jin, Zhou et al., None, J. Med. Virol
Domenech, Pérez, Saldarini, Uad, Musso, None, Int. Urol. Nephrol
Farhangfar, Kurgan, Dy, None, Pattern Recognit
Gibson, None, Am J. Kidney Dis
Gram, Emborg, Schelde, Friis, Nielsen et al., None, PLoS Med
Hughes, Heron, Sterne, Tilling, None, Int. J. Epidemiol
Kale, Shelke, Dagar, Anders, Gaikwad, None, Front. Pharmacol
Portolés, Marques, López-Sánchez, Valdenebro, Munez et al., None, Nephrol. Dial. Transplant
Ren, Luo, Yu, Song, Liang et al., None, Adv. Sci
Sai, Vijeth, Hemalatha, None, J. Assoc. Chest
Services, Common Terminology Criteria for Adverse Events (CTCAE) version 5.0
Shang, Fu, Geng, Zhang, Zhang et al., None, J. Med. Virol
Silver, Beaubien-Souligny, Shah, Harel, Blum et al., None, Kid. Med
Su, Yang, Wan, Yi, Tang et al., None, Kidney Int
Wei, Zeng, Wang, Gui, Zhang et al., None, Front. Pharmacol
Wen, Chen, Tang, Wang, Zhou et al., None, Ann. Med
Wu, Ma, Miao, Ye, Li et al., None, Front. Public Health
Yu, Chang, None, Signal Transduct. Target. Ther
Zhang, Wang, Deng, Liang, Su et al., None, J. Med. Virol
Zhu, None, Frontiers in Pharmacology
DOI record:
{
"DOI": "10.1002/viw.20240075",
"ISSN": [
"2688-3988",
"2688-268X"
],
"URL": "http://dx.doi.org/10.1002/VIW.20240075",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Kidney disease has been the main risk factor of poor prognosis for COVID‐19 patients. The effectiveness and safety of azvudine treatment in COVID‐19 patients with kidney disease have not been reported. Herein, we conducted a nine‐center and retrospective cohort study in China (ClinicalTrials: NCT06349655) that enrolled 32,864 hospitalized COVID‐19 patients, in which 4192 patients were pre‐existed with kidney disease. After exclusions and propensity score matching, a total of 831 kidney disease patients treated with azvudine and 831 kidney disease patients treated without any anti‐viral treatment (normal group) were selected. Based on Kaplan–Meier and Cox regression analysis, we found that azvudine administration had significantly decreased risks of all‐cause death (<jats:italic>p</jats:italic> < 0.0001) and composite disease progression (<jats:italic>p</jats:italic> = 0.012) as compared to the normal group. Multivariate Cox proportional hazards regression analysis demonstrated that the hazard ratio of all‐cause death was 0.64 (95% CI: 0.503–0.826, <jats:italic>p</jats:italic> < 0.001) and the hazard ratio of composite disease progression was 0.81 (95% CI: 0.658–1.004, <jats:italic>p</jats:italic> = 0.05). The subgroup analysis of different characteristics indicated no significant influence of single factor in both all‐cause mortality and composite disease progression. Five sensitivity analyses were employed to verify the robustness of our results. Safety analysis based on adverse event rate demonstrated an increased rate of hypertriglyceridemia after azvudine administration. In conclusion, we are the first to report the effectiveness and safety of azvudine treatment in COVID‐19 patients with kidney disease and demonstrate that azvudine could reduce the risk of all‐cause death without significant adverse events based on a large‐scale, multicenter, retrospective cohort study.</jats:p>",
"alternative-id": [
"10.1002/VIW.20240075"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2024-08-12"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2025-03-10"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2025-04-09"
}
],
"author": [
{
"ORCID": "https://orcid.org/0009-0001-6305-2014",
"affiliation": [
{
"name": "Department of Pharmacy The First Affiliated Hospital of Zhengzhou University Zhengzhou China"
}
],
"authenticated-orcid": false,
"family": "Yu",
"given": "Bo",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases State Key Laboratory of Antiviral Drugs Pingyuan Laboratory The First Affiliated Hospital of Zhengzhou University Zhengzhou China"
}
],
"family": "Yang",
"given": "Mengzhao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases State Key Laboratory of Antiviral Drugs Pingyuan Laboratory The First Affiliated Hospital of Zhengzhou University Zhengzhou China"
}
],
"family": "Yu",
"given": "Jia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases State Key Laboratory of Antiviral Drugs Pingyuan Laboratory The First Affiliated Hospital of Zhengzhou University Zhengzhou China"
}
],
"family": "Wang",
"given": "Daming",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Guangshan County People's Hospital, Guangshan County Xinyang China"
}
],
"family": "Luo",
"given": "Hong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medical Information The First Affiliated Hospital of Zhengzhou University Zhengzhou China"
}
],
"family": "Cheng",
"given": "Ming",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases Shangqiu Municipal Hospital Shangqiu China"
}
],
"family": "Zhang",
"given": "Shixi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases Luoyang Central Hospital Affiliated of Zhengzhou University Luoyang China"
}
],
"family": "Li",
"given": "Guotao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Laboratory Henan Provincial Chest Hospital Affiliated of Zhengzhou University Zhengzhou China"
}
],
"family": "Wang",
"given": "Ling",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastrointestinal Surgery Nanyang Central Hospital Nanyang China"
}
],
"family": "Qian",
"given": "Guowu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases Anyang City Fifth People's Hospital Anyang China"
}
],
"family": "Zhang",
"given": "Donghua",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory and Critical Care Medicine Fengqiu County People's Hospital Xinxiang China"
}
],
"family": "Li",
"given": "Silin",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-0798-3444",
"affiliation": [
{
"name": "Department of Infectious Diseases State Key Laboratory of Antiviral Drugs Pingyuan Laboratory The First Affiliated Hospital of Zhengzhou University Zhengzhou China"
}
],
"authenticated-orcid": false,
"family": "Ren",
"given": "Zhigang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Henan Key Laboratory of Precision Clinical Pharmacy Zhengzhou University Zhengzhou China"
}
],
"family": "Kan",
"given": "Quancheng",
"sequence": "additional"
}
],
"container-title": "VIEW",
"container-title-short": "VIEW",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2025,
4,
9
]
],
"date-time": "2025-04-09T07:25:43Z",
"timestamp": 1744183543000
},
"deposited": {
"date-parts": [
[
2025,
4,
9
]
],
"date-time": "2025-04-09T07:25:51Z",
"timestamp": 1744183551000
},
"funder": [
{
"DOI": "10.13039/501100012166",
"award": [
"2022YFC2303100"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100012166",
"id-type": "DOI"
}
],
"name": "National Key Research and Development Program of China"
}
],
"indexed": {
"date-parts": [
[
2025,
4,
10
]
],
"date-time": "2025-04-10T04:12:16Z",
"timestamp": 1744258336886,
"version": "3.40.4"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
4,
9
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
4,
9
]
],
"date-time": "2025-04-09T00:00:00Z",
"timestamp": 1744156800000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/VIW.20240075",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2025,
4,
9
]
]
},
"published-online": {
"date-parts": [
[
2025,
4,
9
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1002/jmv.25674",
"author": "Zhang N.",
"doi-asserted-by": "crossref",
"first-page": "408",
"journal-title": "J. Med. Virol.",
"key": "e_1_2_10_2_1",
"volume": "92",
"year": "2020"
},
{
"key": "e_1_2_10_3_1",
"unstructured": "World Health Organization https://data.who.int/dashboards/covid19/cases?n=c(accessed: 2024)."
},
{
"DOI": "10.1371/journal.pmed.1003992",
"author": "Gram M.",
"doi-asserted-by": "crossref",
"journal-title": "PLoS Med.",
"key": "e_1_2_10_4_1",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.1016/j.xkme.2020.11.008",
"author": "Silver S.",
"doi-asserted-by": "crossref",
"first-page": "83",
"journal-title": "Kid. Med.",
"key": "e_1_2_10_5_1",
"volume": "3",
"year": "2021"
},
{
"DOI": "10.1016/j.kint.2020.03.005",
"author": "Cheng Y.",
"doi-asserted-by": "crossref",
"first-page": "829",
"journal-title": "Kidney Int.",
"key": "e_1_2_10_6_1",
"volume": "97",
"year": "2020"
},
{
"DOI": "10.1007/s11255-017-1585-z",
"author": "Domenech P.",
"doi-asserted-by": "crossref",
"first-page": "1211",
"journal-title": "Int. Urol. Nephrol.",
"key": "e_1_2_10_7_1",
"volume": "49",
"year": "2017"
},
{
"DOI": "10.1080/07853890.2022.2034936",
"author": "Wen W.",
"doi-asserted-by": "crossref",
"first-page": "516",
"journal-title": "Ann. Med.",
"key": "e_1_2_10_8_1",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.3389/fphar.2023.1228548",
"author": "Zhu K.",
"doi-asserted-by": "crossref",
"journal-title": "Frontiers in Pharmacology",
"key": "e_1_2_10_9_1",
"volume": "14",
"year": "2023"
},
{
"key": "e_1_2_10_10_1",
"unstructured": "National Medical Products Administration homepage http://english.nmpa.gov.cn/2022‐08/15/c_797867.htm(accessed: August 2022)."
},
{
"DOI": "10.1002/advs.202001435",
"author": "Ren Z.",
"doi-asserted-by": "crossref",
"issue": "19",
"journal-title": "Adv. Sci.",
"key": "e_1_2_10_11_1",
"volume": "7",
"year": "2020"
},
{
"key": "e_1_2_10_12_1",
"unstructured": "China N. H. C. o. t. P. s. R. o.COVID‐19 diagnosis and treatment plan (trial version 9).2022.https://www.gov.cn/zhengce/zhengceku/2022‐03/15/5679257/files/49854a49c7004f4ea9e622f3f2c568d8.pdf"
},
{
"key": "e_1_2_10_13_1",
"unstructured": "China N. H. C. o. t. P. s. R. o.COVID‐19 diagnosis and treatment plan (trial version 10).2023.https://www.gov.cn/zhengce/zhengceku/2023‐01/06/5735343/files/5844ce04246b431dbd322d8ba10afb48.pdf"
},
{
"key": "e_1_2_10_14_1",
"unstructured": "SERVICES U. S. D. O. H. A. H.Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.2017.https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf"
},
{
"author": "Sai S.",
"first-page": "59",
"journal-title": "J. Assoc. Chest",
"key": "e_1_2_10_15_1",
"volume": "9",
"year": "2021"
},
{
"author": "Bellocco R.",
"first-page": "63",
"issue": "1",
"journal-title": "Nutrition",
"key": "e_1_2_10_16_1",
"volume": "14",
"year": "1998"
},
{
"DOI": "10.1016/j.patcog.2008.05.019",
"author": "Farhangfar A.",
"doi-asserted-by": "crossref",
"first-page": "3692",
"journal-title": "Pattern Recognit",
"key": "e_1_2_10_17_1",
"volume": "41",
"year": "2008"
},
{
"DOI": "10.1093/ije/dyz032",
"author": "Hughes R.",
"doi-asserted-by": "crossref",
"first-page": "1294",
"journal-title": "Int. J. Epidemiol.",
"key": "e_1_2_10_18_1",
"volume": "48",
"year": "2019"
},
{
"DOI": "10.1093/ndt/gfaa189",
"author": "Portolés J.",
"doi-asserted-by": "crossref",
"first-page": "1353",
"issue": "8",
"journal-title": "Nephrol. Dial. Transplant.",
"key": "e_1_2_10_19_1",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.1007/s11255-020-02740-3",
"author": "Cai R.",
"doi-asserted-by": "crossref",
"first-page": "1623",
"journal-title": "Int. Urol. Nephrol.",
"key": "e_1_2_10_20_1",
"volume": "53",
"year": "2021"
},
{
"DOI": "10.1016/S0272-6386(86)80148-2",
"author": "Gibson T.",
"doi-asserted-by": "crossref",
"first-page": "7",
"issue": "1",
"journal-title": "Am J. Kidney Dis.",
"key": "e_1_2_10_21_1",
"volume": "8",
"year": "1986"
},
{
"DOI": "10.1002/jmv.29007",
"author": "Shang S.",
"doi-asserted-by": "crossref",
"journal-title": "J. Med. Virol.",
"key": "e_1_2_10_22_1",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1016/j.kint.2020.04.003",
"author": "Su H.",
"doi-asserted-by": "crossref",
"first-page": "219",
"journal-title": "Kidney Int.",
"key": "e_1_2_10_23_1",
"volume": "98",
"year": "2020"
},
{
"DOI": "10.1038/s41392-020-00351-z",
"author": "Yu B.",
"doi-asserted-by": "crossref",
"first-page": "236",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "e_1_2_10_24_1",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.3389/fphar.2023.1274294",
"author": "Wei A.",
"doi-asserted-by": "crossref",
"journal-title": "Front. Pharmacol.",
"key": "e_1_2_10_25_1",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1002/jmv.28756",
"author": "Deng G.",
"doi-asserted-by": "crossref",
"journal-title": "J. Med. Virol.",
"key": "e_1_2_10_26_1",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.3389/fphar.2023.1053814",
"author": "Kale A.",
"doi-asserted-by": "crossref",
"journal-title": "Front. Pharmacol.",
"key": "e_1_2_10_27_1",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.3389/fpubh.2022.796467",
"author": "Wu J.",
"doi-asserted-by": "crossref",
"journal-title": "Front. Public Health",
"key": "e_1_2_10_28_1",
"volume": "10",
"year": "2022"
}
],
"reference-count": 27,
"references-count": 27,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/VIW.20240075"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "The effectiveness and safety of azvudine treatment in COVID‐19 patients with kidney disease based on a multicenter retrospective cohort study",
"type": "journal-article",
"update-policy": "https://doi.org/10.1002/crossmark_policy"
}