Famotidine Use Is Not Associated With 30-day Mortality: A Coarsened Exact Match Study in 7158 Hospitalized Patients With Coronavirus Disease 2019 From a Large Healthcare System
et al., Gastroenterology, doi:10.1053/j.gastro.2020.10.011, Feb 2021
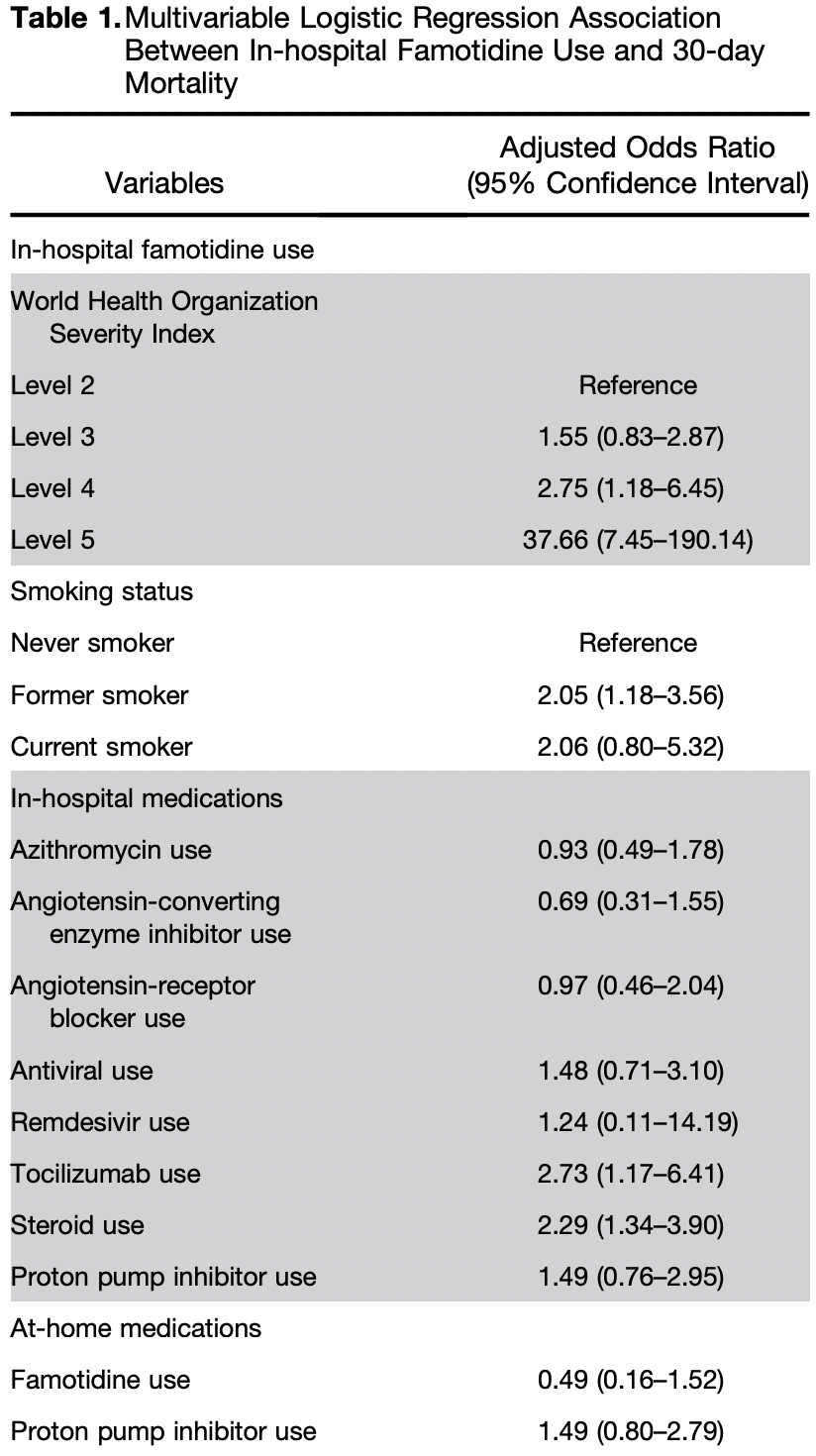
Retrospective 7,158 hospitalized COVID-19 patients in the USA analyzing famotidine treatment, showing no significant difference in mortality with associated remdesivir treatment.
Gérard, Zhou, Wu, Kamo, Choi, Kim show increased risk of acute kidney injury, Leo, Briciu, Muntean, Petrov show increased risk of liver injury, and Negru, Cheng, Mohammed, Kwok show increased risk of cardiac disorders with remdesivir.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments15.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
Study covers remdesivir and famotidine.
|
risk of death, 24.0% higher, OR 1.24, p = 0.87, treatment 32, control 7,126, adjusted per study, multivariable, day 30, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Gérard et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145.
2.
Zhou et al., Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679.
3.
Wu et al., Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS, Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828.
4.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
5.
Choi et al., Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0.
6.
Kim et al., Investigating the Safety Profile of Fast‐Track COVID‐19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study, Pharmacoepidemiology and Drug Safety, doi:10.1002/pds.70043.
7.
Leo et al., Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points, Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014.
8.
Briciu et al., Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting, Pathogens, doi:10.3390/pathogens12121453.
9.
Muntean et al., Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre, Pharmaceuticals, doi:10.3390/ph17010003.
10.
Petrov et al., The Effect of Potentially Hepatotoxic Medicinal Products on Alanine Transaminase Levels in COVID-19 Patients: A Case–Control Study, Safety and Risk of Pharmacotherapy, doi:10.30895/2312-7821-2025-458.
11.
Negru et al., Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data, Biomedicines, doi:10.3390/biomedicines13061387.
12.
Cheng et al., Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase, Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z.
13.
Mohammed et al., Bradycardia associated with remdesivir treatment in coronavirus disease 2019 patients: A propensity score-matched analysis, Medicine, doi:10.1097/MD.0000000000044501.
Yeramaneni et al., 28 Feb 2021, retrospective, USA, peer-reviewed, 6 authors, study period 11 February, 2020 - 8 May, 2020.
Famotidine Use Is Not Associated With 30-day Mortality: A Coarsened Exact Match Study in 7158 Hospitalized Patients With Coronavirus Disease 2019 From a Large Healthcare System
Gastroenterology, doi:10.1053/j.gastro.2020.10.011
P revious reports have found that in-hospital famotidine use in coronavirus disease 2019 (COVID-19) patients was associated with reduced risk of death or intubation. 1, 2 In 1 of these studies the authors proposed that famotidine inhibits the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) protease, 3-chymotrypsin-like protease, that is essential for breakdown of the immature SARS-CoV-2 protein particles that contribute to the inflammatory response seen in some COVID-19-infected individuals, 1 which in turn can lead to acute respiratory distress syndrome, multiorgan dysfunction, physiologic deterioration, and death. 3 In a global pandemic with a lack of US Food and Drug Administration-approved targeted therapeutic agents, identification and repurposing of well-established drugs with a proven track record of safety, affordability, and widespread availability are necessary. 4 The purpose of this study was to evaluate the reported protective effect of famotidine on mortality in hospitalized COVID-19 patients.
Supplementary Material Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org and at https://doi.org/10.1053/ j.gastro.2020.10.011.
Conflicts of interest The authors disclose no conflicts.
References
Dr, Luther, Boulevard, Suite 800
Freedberg, None, Gastroenterology
Iacus, None, Political Analysis
Mather, None, Am J Gastroenterol
Rogosnitzky, None, JMIR Public Health Surveill
Wang, None, Lancet
Zhou, None, Lancet
DOI record:
{
"DOI": "10.1053/j.gastro.2020.10.011",
"ISSN": [
"0016-5085"
],
"URL": "http://dx.doi.org/10.1053/j.gastro.2020.10.011",
"alternative-id": [
"S0016508520352495"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Famotidine Use Is Not Associated With 30-day Mortality: A Coarsened Exact Match Study in 7158 Hospitalized Patients With Coronavirus Disease 2019 From a Large Healthcare System"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Gastroenterology"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1053/j.gastro.2020.10.011"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 by the AGA Institute"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-1411-2359",
"affiliation": [],
"authenticated-orcid": false,
"family": "Yeramaneni",
"given": "Samrat",
"sequence": "first"
},
{
"affiliation": [],
"family": "Doshi",
"given": "Pratik",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sands",
"given": "Kenneth",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cooper",
"given": "Mandelin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kurbegov",
"given": "Dax",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fromell",
"given": "Gregg",
"sequence": "additional"
}
],
"container-title": [
"Gastroenterology"
],
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.jp",
"clinicalkey.com",
"gastrojournal.org",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.fr",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2020,
10,
12
]
],
"date-time": "2020-10-12T05:38:45Z",
"timestamp": 1602481125000
},
"deposited": {
"date-parts": [
[
2021,
3,
20
]
],
"date-time": "2021-03-20T07:51:40Z",
"timestamp": 1616226700000
},
"funder": [
{
"DOI": "10.13039/100016646",
"doi-asserted-by": "publisher",
"name": "HCA Healthcare"
}
],
"indexed": {
"date-parts": [
[
2021,
12,
23
]
],
"date-time": "2021-12-23T22:27:20Z",
"timestamp": 1640298440528
},
"is-referenced-by-count": 17,
"issn-type": [
{
"type": "print",
"value": "0016-5085"
}
],
"issue": "3",
"issued": {
"date-parts": [
[
2021,
2
]
]
},
"journal-issue": {
"issue": "3",
"published-print": {
"date-parts": [
[
2021,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
2,
1
]
],
"date-time": "2021-02-01T00:00:00Z",
"timestamp": 1612137600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0016508520352495?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0016508520352495?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "919-921.e3",
"prefix": "10.1053",
"published": {
"date-parts": [
[
2021,
2
]
]
},
"published-print": {
"date-parts": [
[
2021,
2
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1053/j.gastro.2020.05.053",
"author": "Freedberg",
"doi-asserted-by": "crossref",
"first-page": "1129",
"journal-title": "Gastroenterology",
"key": "10.1053/j.gastro.2020.10.011_bib1",
"volume": "159",
"year": "2020"
},
{
"DOI": "10.14309/ajg.0000000000000832",
"author": "Mather",
"doi-asserted-by": "crossref",
"first-page": "1617",
"journal-title": "Am J Gastroenterol",
"key": "10.1053/j.gastro.2020.10.011_bib2",
"volume": "115",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"journal-title": "Lancet",
"key": "10.1053/j.gastro.2020.10.011_bib3",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.2196/19199",
"author": "Rogosnitzky",
"doi-asserted-by": "crossref",
"journal-title": "JMIR Public Health Surveill",
"key": "10.1053/j.gastro.2020.10.011_bib4",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1093/pan/mpr013",
"author": "Iacus",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Political Analysis",
"key": "10.1053/j.gastro.2020.10.011_bib5",
"volume": "20",
"year": "2012"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1569",
"journal-title": "Lancet",
"key": "10.1053/j.gastro.2020.10.011_bib6",
"volume": "395",
"year": "2020"
},
{
"journal-title": "N Engl J Med",
"key": "10.1053/j.gastro.2020.10.011_bib7",
"year": "2020"
}
],
"reference-count": 7,
"references-count": 7,
"relation": {},
"score": 1,
"short-container-title": [
"Gastroenterology"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Gastroenterology",
"Hepatology"
],
"subtitle": [],
"title": [
"Famotidine Use Is Not Associated With 30-day Mortality: A Coarsened Exact Match Study in 7158 Hospitalized Patients With Coronavirus Disease 2019 From a Large Healthcare System"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "160"
}
yeramaneni