Impact of early and delayed azvudine administration on COVID-19 mortality: a retrospective study
et al., Scientific Reports, doi:10.1038/s41598-025-05381-7, Jul 2025
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.0000000041 from 40 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
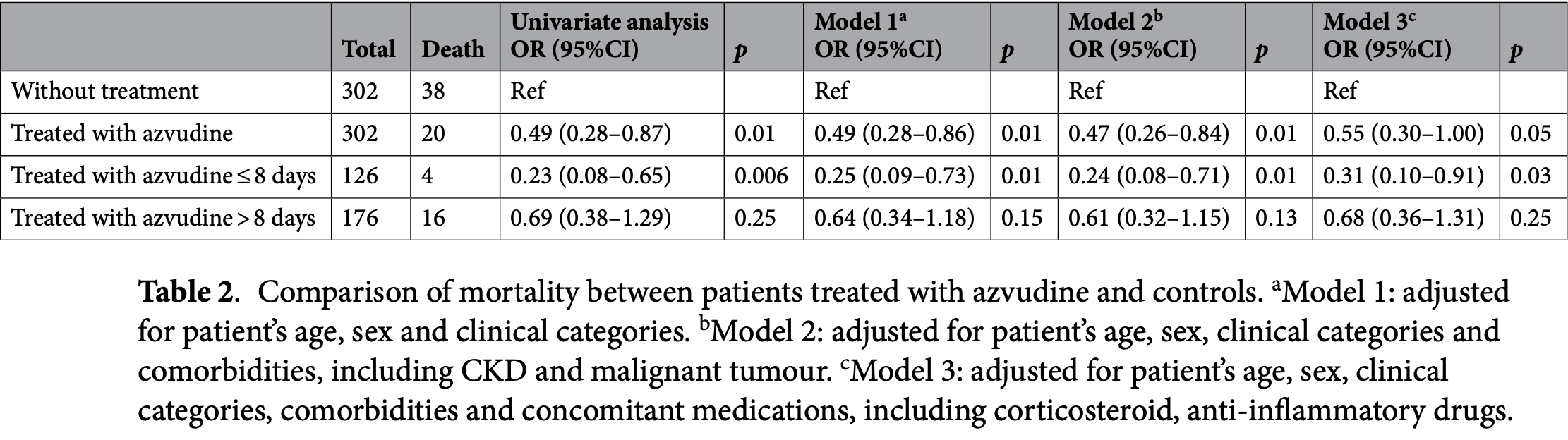
PSM retrospective 604 COVID-19 patients (302 receiving azvudine, 302 controls) showing reduced mortality with azvudine treatment when administered within 8 days of symptom onset.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments3.
|
risk of death, 45.0% lower, OR 0.55, p = 0.05, treatment 302, control 302, all patients, RR approximated with OR.
|
|
risk of death, 69.0% lower, OR 0.31, p = 0.03, treatment 126, control 302, ≤ 8 days, RR approximated with OR.
|
|
risk of death, 32.0% lower, OR 0.68, p = 0.25, treatment 176, control 302, > 8 days, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Xiong et al., Real-world data of Azvudine-induced hepatotoxicity among hospitalized COVID-19 patients in China: a retrospective case-control study, Frontiers in Pharmacology, doi:10.3389/fphar.2025.1558054.
Wang et al., 1 Jul 2025, retrospective, China, peer-reviewed, 27 authors, study period 1 November, 2022 - 27 February, 2023.
Contact: tianxl@pumch.cn, mengzhaowang@sina.com, shuyangzhang103@nrdrs.org.
Impact of early and delayed azvudine administration on COVID-19 mortality: a retrospective study
Scientific Reports, doi:10.1038/s41598-025-05381-7
Azvudine is recommended for patients with coronavirus disease 19 (COVID-19); however, its optimum therapeutic time window and its impact on mortality of patients are unclear. This single-centre, retrospective study from 1 November 2022 to 27 February 2023 conducted at the Peking Union Medical College Hospital was to discuss the dosing window of azvudine and compare the prognostic impact on COVID-19 patients of azvudine use within and after the defined time window. Therapeutic time window referred to the time interval between the onset of the disease and the drug administration. 28day all-cause mortality and the incidence of 28-day disease progression were assessed using univariate logistic regression and adjusted for covariates through multivariate logistic regression analysis. A total of 421 COVID-19 patients using azvudine and 720 patients not using any anti-SARS-CoV-2 drugs were enrolled. After propensity score matching, 302 patients treated with azvudine and 302 patients without antiviral drugs were included. Multivariate logistic regression analysis showed that the use of azvudine was significantly protective until 8 days of symptom onset for COVID-19 patients. Compared with the latter, treatment with azvudine reduced the all-cause mortality rate (OR 0.55, 95% CI 0.30-1.00) and disease progression rate (OR 0.52, 95% CI 0.29-0.93) to 28 days. The study indicated that the benefit of azvudine seemed more significant within 8 days of symptoms onset and the administration of azvudine reduced the risk of death in adult COVID-19 patients. In the future, large randomized controlled trials (RCT) studies are needed to confirm our conclusions because of the inherent limitation of single-centre, retrospective study.
Author contributions L.W., J.F., Y.X., X.T., M.W. and S.Z. designed the study. Y.W., C.C. and H.X. did the analyses. L.W. and Y.W. drafted the manuscript. The other authors revised the initial draft. All authors approved the final version of the manuscript.
Declarations
Ethics approval and consent to participate This study was approved by the Institutional Review Board of Peking Union Medical College Hospital (I-23PJ946). As this was a retrospective study, the requirement for informed consent was waived by Institutional Review Board of Peking Union Medical College Hospital.
Competing interests The authors declare no competing interests.
References
Arbel, Nirmatrelvir use and severe Covid-19 outcomes during the Omicron surge, N. Engl. J. Med, doi:10.1056/NEJMoa2204919
Austin, Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research, Commun. Stat. Simul. Comput, doi:10.1080/03610910902859574
Barghash, In Silico modeling as a perspective in developing potential vaccine candidates and therapeutics for COVID-19, Coatings
Barghash, Navigating the COVID-19 therapeutic landscape: unveiling novel perspectives on FDA-Approved medications, vaccination targets, and emerging novel strategies, Molecules, doi:10.3390/molecules29235564
Chandrasekar, Mohammad, Aboumarzouk, Singh, Dakua, Quantitative prediction of toxicological points of departure using two-stage machine learning models: A new approach methodology (NAM) for chemical risk assessment, J. Hazard. Mater, doi:10.1016/j.jhazmat.2024.137071
Deng, Real-world effectiveness of azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: A retrospective cohort study, J. Med. Virol, doi:10.1002/jmv.28756
Docherty, Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study, BMJ, doi:10.1136/bmj.m1985
Gottlieb, Early Remdesivir to prevent progression to severe Covid-19 in outpatients, N. Engl. J. Med, doi:10.1056/NEJMoa2116846
Guan, Clinical characteristics of coronavirus disease 2019 in China, N Engl. J. Med, doi:10.1056/NEJMoa2002032
Hammond, Oral nirmatrelvir for High-Risk, nonhospitalized adults with Covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2118542
Kabinger, Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis, Nat. Struct. Mol. Biol, doi:10.1038/s41594-021-00651-0
Lamontagne, A living WHO guideline on drugs for covid-19, BMJ, doi:10.1136/bmj.m3379
Li, Association of nirmatrelvir/ritonavir treatment on upper respiratory severe acute respiratory syndrome coronavirus 2 reverse Transcription-Polymerase chain reaction (SARS-Cov-2 RT-PCR) negative conversion rates among High-Risk patients with coronavirus disease 2019 (COVID-19), Clin. Infect. Dis, doi:10.1093/cid/ciac600
Li, Hilgenfeld, Whitley, De Clercq, Therapeutic strategies for COVID-19: progress and lessons learned, Nat. Rev. Drug Discov, doi:10.1038/s41573-023-00672-y
Markovskaya, Gavioli, Cusumano, Glatt, Coronavirus disease 2019 (COVID-19): secondary bacterial infections and the impact on antimicrobial resistance during the COVID-19 pandemic, Antimicrob. Steward Healthc. Epidemiol, doi:10.1017/ash.2022.253
Meslé, Estimated number of lives directly saved by COVID-19 vaccination programmes in the WHO European region from december, 2020, to march, 2023: a retrospective surveillance study, Lancet Respir Med, doi:10.1016/s2213-2600(24)00179-6
Poduri, Joshi, Jagadeesh, Drugs targeting various stages of the SARS-CoV-2 life cycle: exploring promising drugs for the treatment of Covid-19, Cell. Signal, doi:10.1016/j.cellsig.2020.109721
Ren, Open-Label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study, Adv. Sci. (Weinh), doi:10.1002/advs.202001435
Saravolatz, Depcinski, Sharma, Molnupiravir and Nirmatrelvir-Ritonavir: oral coronavirus disease 2019 antiviral drugs, Clin. Infect. Dis, doi:10.1093/cid/ciac180
Sheng, Selectively T cell phosphorylation activation of azvudine in the thymus tissue with immune protection effect, Acta Pharm. Sin B, doi:10.1016/j.apsb.2024.03.032
Singh, Investigating tattoo pigments composition with UV-Vis and FT-IR spectroscopy supported by chemometric modelling, Curr. Anal. Chem, doi:10.2174/0115734110316443240725051037
Sun, Oral azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study, EClinicalMedicine, doi:10.1016/j.eclinm.2023.101981
Tian, Efficacy and safety of azvudine in symptomatic adult COVID-19 participants who are at increased risk of progressing to critical illness: a study protocol for a multicentre randomized double-blind placebo-controlled phase III trial, Trials, doi:10.1186/s13063-024-07914-3
Tian, Sun, Xu, Ye, The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant, J. Med. Virol, doi:10.1002/jmv.27643
Von Elm, The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies, Lancet, doi:10.1016/s0140-6736(07)61602-x
Wai, Association of molnupiravir and Nirmatrelvir-Ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19, Lancet Reg. Health West. Pac, doi:10.1016/j.lanwpc.2022.100602
Wong, Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong kong's Omicron BA.2 wave: a retrospective cohort study, Lancet Infect. Dis, doi:10.1016/s1473-3099(22)00507-2
Wong, Real-world effectiveness of molnupiravir and nirmatrelvir plus Ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the Omicron wave in Hong kong: an observational study, Lancet, doi:10.1016/s0140-6736(22)01586-0
Xie, Effectiveness and safety of azvudine versus nirmatrelvir-ritonavir in adult patients infected with COVID-19 Omicron strains: a retrospective study in Beijing, Sci. Rep, doi:10.1038/s41598-024-74502-5
Yu, Chang, The first Chinese oral anti-COVID-19 drug azvudine launched, Innov. (Camb), doi:10.1016/j.xinn.2022.100321
Zhang, Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients, Signal. Transduct. Target. Ther, doi:10.1038/s41392-021-00835-6
Zhang, Kim, Lonjon, Zhu, Balance diagnostics after propensity score matching, Ann. Transl Med, doi:10.21037/atm.2018.12.10
Zhou, Zhi, Teng, The outbreak of SARS-CoV-2 Omicron lineages, immune escape, and vaccine effectivity, J. Med. Virol, doi:10.1002/jmv.28138
DOI record:
{
"DOI": "10.1038/s41598-025-05381-7",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-025-05381-7",
"alternative-id": [
"5381"
],
"article-number": "21729",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "14 August 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2 June 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "1 July 2025"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "This study was approved by the Institutional Review Board of Peking Union Medical College Hospital (I-23PJ946). As this was a retrospective study, the requirement for informed consent was waived by Institutional Review Board of Peking Union Medical College Hospital."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Wang",
"given": "Luo",
"sequence": "first"
},
{
"affiliation": [],
"family": "Wang",
"given": "Yaqi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xu",
"given": "Yan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fan",
"given": "Junping",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pan",
"given": "Siqi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xie",
"given": "Huaiya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Ting",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Ruxuan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Xiaoyan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shi",
"given": "Chuan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhou",
"given": "Qing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shi",
"given": "Yuequan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cheng",
"given": "Chongsheng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dai",
"given": "Lu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Haoran",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wu",
"given": "Aohua",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Xueqi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Hong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Jinglan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Du",
"given": "Bin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Jihai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhu",
"given": "Huadong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ma",
"given": "Xiaojun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huang",
"given": "Jing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tian",
"given": "Xinlun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Mengzhao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Shuyang",
"sequence": "additional"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
7,
1
]
],
"date-time": "2025-07-01T18:38:47Z",
"timestamp": 1751395127000
},
"deposited": {
"date-parts": [
[
2025,
7,
1
]
],
"date-time": "2025-07-01T18:38:49Z",
"timestamp": 1751395129000
},
"funder": [
{
"award": [
"2021-I2M-1-048"
],
"name": "Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences"
},
{
"award": [
"2023YFC3041900"
],
"name": "National Key Research and Development Program of China"
},
{
"award": [
"2023-PUMCH-G-001"
],
"name": "National High Level Hospital Clinical Research Funding"
}
],
"indexed": {
"date-parts": [
[
2025,
7,
1
]
],
"date-time": "2025-07-01T19:10:05Z",
"timestamp": 1751397005388,
"version": "3.41.0"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2025,
7,
1
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2025,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
7,
1
]
],
"date-time": "2025-07-01T00:00:00Z",
"timestamp": 1751328000000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
7,
1
]
],
"date-time": "2025-07-01T00:00:00Z",
"timestamp": 1751328000000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-025-05381-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-025-05381-7",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-025-05381-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2025,
7,
1
]
]
},
"published-online": {
"date-parts": [
[
2025,
7,
1
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "5381_CR1",
"unstructured": "WHO COVID-19 Dashboard. Geneva: World Health Organization, 2020 (2023). https://covid19.who.int/"
},
{
"DOI": "10.1002/jmv.27643",
"author": "D Tian",
"doi-asserted-by": "publisher",
"first-page": "2376",
"journal-title": "J. Med. Virol.",
"key": "5381_CR2",
"unstructured": "Tian, D., Sun, Y., Xu, H. & Ye, Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J. Med. Virol. 94, 2376–2383. https://doi.org/10.1002/jmv.27643 (2022).",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.1002/jmv.28138",
"author": "Y Zhou",
"doi-asserted-by": "publisher",
"first-page": "e28138",
"journal-title": "J. Med. Virol.",
"key": "5381_CR3",
"unstructured": "Zhou, Y., Zhi, H. & Teng, Y. The outbreak of SARS-CoV-2 Omicron lineages, immune escape, and vaccine effectivity. J. Med. Virol. 95, e28138. https://doi.org/10.1002/jmv.28138 (2023).",
"volume": "95",
"year": "2023"
},
{
"key": "5381_CR4",
"unstructured": "Joint prevention and control Mechnism of The State Council for the Novel Coronavirus Pneumonia. Notice on further optimizing and implementing the prevention and control measures of the novel coronavirus (2022). http://www.gov.cn/xinwen/2022-12/07/content_5730443.htm"
},
{
"DOI": "10.3390/molecules29235564",
"doi-asserted-by": "publisher",
"key": "5381_CR5",
"unstructured": "Barghash, R. F. et al. Navigating the COVID-19 therapeutic landscape: unveiling novel perspectives on FDA-Approved medications, vaccination targets, and emerging novel strategies. Molecules. 29 https://doi.org/10.3390/molecules29235564 (2024)."
},
{
"DOI": "10.3390/coatings11111273",
"author": "RF Barghash",
"doi-asserted-by": "publisher",
"first-page": "1273",
"journal-title": "Coatings",
"key": "5381_CR6",
"unstructured": "Barghash, R. F. et al. In Silico modeling as a perspective in developing potential vaccine candidates and therapeutics for COVID-19. Coatings. 11, 1273 (2021).",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1136/bmj.m3379",
"author": "F Lamontagne",
"doi-asserted-by": "publisher",
"first-page": "m3379",
"journal-title": "BMJ",
"key": "5381_CR7",
"unstructured": "Lamontagne, F. et al. A living WHO guideline on drugs for covid-19. BMJ. 370, m3379. https://doi.org/10.1136/bmj.m3379 (2020).",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1016/j.lanwpc.2022.100602",
"author": "AK Wai",
"doi-asserted-by": "publisher",
"first-page": "100602",
"journal-title": "Lancet Reg. Health West. Pac.",
"key": "5381_CR8",
"unstructured": "Wai, A. K. et al. Association of molnupiravir and Nirmatrelvir-Ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19. Lancet Reg. Health West. Pac. 30, 100602. https://doi.org/10.1016/j.lanwpc.2022.100602 (2023).",
"volume": "30",
"year": "2023"
},
{
"DOI": "10.1016/s1473-3099(22)00507-2",
"author": "CKH Wong",
"doi-asserted-by": "publisher",
"first-page": "1681",
"journal-title": "Lancet Infect. Dis.",
"key": "5381_CR9",
"unstructured": "Wong, C. K. H. et al. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong kong’s Omicron BA.2 wave: a retrospective cohort study. Lancet Infect. Dis. 22, 1681–1693. https://doi.org/10.1016/s1473-3099(22)00507-2 (2022).",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1016/s0140-6736(22)01586-0",
"author": "CKH Wong",
"doi-asserted-by": "publisher",
"first-page": "1213",
"journal-title": "Lancet",
"key": "5381_CR10",
"unstructured": "Wong, C. K. H. et al. Real-world effectiveness of molnupiravir and nirmatrelvir plus Ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the Omicron wave in Hong kong: an observational study. Lancet 400, 1213–1222. https://doi.org/10.1016/s0140-6736(22)01586-0 (2022).",
"volume": "400",
"year": "2022"
},
{
"DOI": "10.1016/j.xinn.2022.100321",
"author": "B Yu",
"doi-asserted-by": "publisher",
"first-page": "100321",
"journal-title": "Innov. (Camb)",
"key": "5381_CR11",
"unstructured": "Yu, B. & Chang, J. The first Chinese oral anti-COVID-19 drug azvudine launched. Innov. (Camb). 3, 100321. https://doi.org/10.1016/j.xinn.2022.100321 (2022).",
"volume": "3",
"year": "2022"
},
{
"key": "5381_CR12",
"unstructured": "Diagnosis and treatment protocol for COVID-19 in China. (trial version 10). (2023). https://www.gov.cn/zhengce/zhengceku/2023-01/06/content_5735343.htm"
},
{
"DOI": "10.1002/advs.202001435",
"author": "Z Ren",
"doi-asserted-by": "publisher",
"first-page": "e2001435",
"journal-title": "Adv. Sci. (Weinh)",
"key": "5381_CR13",
"unstructured": "Ren, Z. et al. Open-Label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study. Adv. Sci. (Weinh). 7, e2001435. https://doi.org/10.1002/advs.202001435 (2020). A Randomized.",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1038/s41392-021-00835-6",
"author": "JL Zhang",
"doi-asserted-by": "publisher",
"first-page": "414",
"journal-title": "Signal. Transduct. Target. Ther.",
"key": "5381_CR14",
"unstructured": "Zhang, J. L. et al. Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal. Transduct. Target. Ther. 6, 414. https://doi.org/10.1038/s41392-021-00835-6 (2021).",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1002/jmv.28756",
"author": "G Deng",
"doi-asserted-by": "publisher",
"first-page": "e28756",
"journal-title": "J. Med. Virol.",
"key": "5381_CR15",
"unstructured": "Deng, G. et al. Real-world effectiveness of azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: A retrospective cohort study. J. Med. Virol. 95, e28756. https://doi.org/10.1002/jmv.28756 (2023).",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1016/j.eclinm.2023.101981",
"author": "Y Sun",
"doi-asserted-by": "publisher",
"first-page": "101981",
"journal-title": "EClinicalMedicine",
"key": "5381_CR16",
"unstructured": "Sun, Y. et al. Oral azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study. EClinicalMedicine. 59, 101981. https://doi.org/10.1016/j.eclinm.2023.101981 (2023).",
"volume": "59",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2204919",
"author": "R Arbel",
"doi-asserted-by": "publisher",
"first-page": "790",
"journal-title": "N. Engl. J. Med.",
"key": "5381_CR17",
"unstructured": "Arbel, R. et al. Nirmatrelvir use and severe Covid-19 outcomes during the Omicron surge. N. Engl. J. Med. 387, 790–798. https://doi.org/10.1056/NEJMoa2204919 (2022).",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"author": "J Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"journal-title": "N. Engl. J. Med.",
"key": "5381_CR18",
"unstructured": "Hammond, J. et al. Oral nirmatrelvir for High-Risk, nonhospitalized adults with Covid-19. N. Engl. J. Med. 386, 1397–1408. https://doi.org/10.1056/NEJMoa2118542 (2022).",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac600",
"author": "H Li",
"doi-asserted-by": "publisher",
"first-page": "e148",
"journal-title": "Clin. Infect. Dis.",
"key": "5381_CR19",
"unstructured": "Li, H. et al. Association of nirmatrelvir/ritonavir treatment on upper respiratory severe acute respiratory syndrome coronavirus 2 reverse Transcription-Polymerase chain reaction (SARS-Cov-2 RT-PCR) negative conversion rates among High-Risk patients with coronavirus disease 2019 (COVID-19). Clin. Infect. Dis. 76, e148–e154. https://doi.org/10.1093/cid/ciac600 (2023).",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac180",
"author": "LD Saravolatz",
"doi-asserted-by": "publisher",
"first-page": "165",
"journal-title": "Clin. Infect. Dis.",
"key": "5381_CR20",
"unstructured": "Saravolatz, L. D., Depcinski, S. & Sharma, M. Molnupiravir and Nirmatrelvir-Ritonavir: oral coronavirus disease 2019 antiviral drugs. Clin. Infect. Dis. 76, 165–171. https://doi.org/10.1093/cid/ciac180 (2023).",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2116846",
"author": "RL Gottlieb",
"doi-asserted-by": "publisher",
"first-page": "305",
"journal-title": "N. Engl. J. Med.",
"key": "5381_CR21",
"unstructured": "Gottlieb, R. L. et al. Early Remdesivir to prevent progression to severe Covid-19 in outpatients. N. Engl. J. Med. 386, 305–315. https://doi.org/10.1056/NEJMoa2116846 (2022).",
"volume": "386",
"year": "2022"
},
{
"key": "5381_CR22",
"unstructured": "Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0, < (2017). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf."
},
{
"DOI": "10.1016/j.cellsig.2020.109721",
"author": "R Poduri",
"doi-asserted-by": "publisher",
"first-page": "109721",
"journal-title": "Cell. Signal.",
"key": "5381_CR23",
"unstructured": "Poduri, R., Joshi, G. & Jagadeesh, G. Drugs targeting various stages of the SARS-CoV-2 life cycle: exploring promising drugs for the treatment of Covid-19. Cell. Signal. 74, 109721. https://doi.org/10.1016/j.cellsig.2020.109721 (2020).",
"volume": "74",
"year": "2020"
},
{
"DOI": "10.1016/j.apsb.2024.03.032",
"author": "N Sheng",
"doi-asserted-by": "publisher",
"first-page": "3140",
"journal-title": "Acta Pharm. Sin B",
"key": "5381_CR24",
"unstructured": "Sheng, N. et al. Selectively T cell phosphorylation activation of azvudine in the thymus tissue with immune protection effect. Acta Pharm. Sin B. 14, 3140–3154. https://doi.org/10.1016/j.apsb.2024.03.032 (2024).",
"volume": "14",
"year": "2024"
},
{
"DOI": "10.1038/s41573-023-00672-y",
"author": "G Li",
"doi-asserted-by": "publisher",
"first-page": "449",
"journal-title": "Nat. Rev. Drug Discov",
"key": "5381_CR25",
"unstructured": "Li, G., Hilgenfeld, R., Whitley, R. & De Clercq, E. Therapeutic strategies for COVID-19: progress and lessons learned. Nat. Rev. Drug Discov. 22, 449–475. https://doi.org/10.1038/s41573-023-00672-y (2023).",
"volume": "22",
"year": "2023"
},
{
"DOI": "10.1038/s41594-021-00651-0",
"author": "F Kabinger",
"doi-asserted-by": "publisher",
"first-page": "740",
"journal-title": "Nat. Struct. Mol. Biol.",
"key": "5381_CR26",
"unstructured": "Kabinger, F. et al. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat. Struct. Mol. Biol. 28, 740–746. https://doi.org/10.1038/s41594-021-00651-0 (2021).",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1038/s41598-024-74502-5",
"author": "H Xie",
"doi-asserted-by": "publisher",
"first-page": "23974",
"journal-title": "Sci. Rep.",
"key": "5381_CR27",
"unstructured": "Xie, H. et al. Effectiveness and safety of azvudine versus nirmatrelvir-ritonavir in adult patients infected with COVID-19 Omicron strains: a retrospective study in Beijing. Sci. Rep. 14, 23974. https://doi.org/10.1038/s41598-024-74502-5 (2024).",
"volume": "14",
"year": "2024"
},
{
"DOI": "10.1016/j.jhazmat.2024.137071",
"author": "V Chandrasekar",
"doi-asserted-by": "publisher",
"first-page": "137071",
"journal-title": "J. Hazard. Mater.",
"key": "5381_CR28",
"unstructured": "Chandrasekar, V., Mohammad, S., Aboumarzouk, O., Singh, A. V. & Dakua, S. P. Quantitative prediction of toxicological points of departure using two-stage machine learning models: A new approach methodology (NAM) for chemical risk assessment. J. Hazard. Mater. 487, 137071. https://doi.org/10.1016/j.jhazmat.2024.137071 (2025).",
"volume": "487",
"year": "2025"
},
{
"DOI": "10.2174/0115734110316443240725051037",
"doi-asserted-by": "publisher",
"key": "5381_CR29",
"unstructured": "Singh, A. et al. Investigating tattoo pigments composition with UV-Vis and FT-IR spectroscopy supported by chemometric modelling. Curr. Anal. Chem. 20 https://doi.org/10.2174/0115734110316443240725051037 (2024)."
},
{
"DOI": "10.1056/NEJMoa2002032",
"author": "WJ Guan",
"doi-asserted-by": "publisher",
"first-page": "1708",
"journal-title": "N. Engl. J. Med.",
"key": "5381_CR30",
"unstructured": "Guan, W. J. et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl. J. Med. 382, 1708–1720. https://doi.org/10.1056/NEJMoa2002032 (2020).",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m1985",
"doi-asserted-by": "publisher",
"key": "5381_CR31",
"unstructured": "Docherty, A. B. et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 369, m1985. https://doi.org/10.1136/bmj.m1985 (2020)."
},
{
"DOI": "10.1016/s2213-2600(24)00179-6",
"author": "MMI Meslé",
"doi-asserted-by": "publisher",
"first-page": "714",
"journal-title": "Lancet Respir Med.",
"key": "5381_CR32",
"unstructured": "Meslé, M. M. I. et al. Estimated number of lives directly saved by COVID-19 vaccination programmes in the WHO European region from december, 2020, to march, 2023: a retrospective surveillance study. Lancet Respir Med. 12, 714–727. https://doi.org/10.1016/s2213-2600(24)00179-6 (2024).",
"volume": "12",
"year": "2024"
},
{
"DOI": "10.1017/ash.2022.253",
"author": "Y Markovskaya",
"doi-asserted-by": "publisher",
"first-page": "e114",
"journal-title": "Antimicrob. Steward Healthc. Epidemiol.",
"key": "5381_CR33",
"unstructured": "Markovskaya, Y., Gavioli, E. M., Cusumano, J. A. & Glatt, A. E. Coronavirus disease 2019 (COVID-19): secondary bacterial infections and the impact on antimicrobial resistance during the COVID-19 pandemic. Antimicrob. Steward Healthc. Epidemiol. 2, e114. https://doi.org/10.1017/ash.2022.253 (2022).",
"volume": "2",
"year": "2022"
},
{
"DOI": "10.1186/s13063-024-07914-3",
"author": "X Tian",
"doi-asserted-by": "publisher",
"first-page": "77",
"journal-title": "Trials",
"key": "5381_CR34",
"unstructured": "Tian, X. et al. Efficacy and safety of azvudine in symptomatic adult COVID-19 participants who are at increased risk of progressing to critical illness: a study protocol for a multicentre randomized double-blind placebo-controlled phase III trial. Trials 25, 77. https://doi.org/10.1186/s13063-024-07914-3 (2024).",
"volume": "25",
"year": "2024"
},
{
"DOI": "10.1016/s0140-6736(07)61602-x",
"author": "E von Elm",
"doi-asserted-by": "publisher",
"first-page": "1453",
"journal-title": "Lancet",
"key": "5381_CR35",
"unstructured": "von Elm, E. et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370, 1453–1457. https://doi.org/10.1016/s0140-6736(07)61602-x (2007).",
"volume": "370",
"year": "2007"
},
{
"DOI": "10.21037/atm.2018.12.10",
"author": "Z Zhang",
"doi-asserted-by": "publisher",
"first-page": "16",
"journal-title": "Ann. Transl. Med.",
"key": "5381_CR36",
"unstructured": "Zhang, Z., Kim, H. J., Lonjon, G. & Zhu, Y. Balance diagnostics after propensity score matching. Ann. Transl Med. 7, 16. https://doi.org/10.21037/atm.2018.12.10 (2019).",
"volume": "7",
"year": "2019"
},
{
"DOI": "10.1080/03610910902859574",
"author": "PC Austin",
"doi-asserted-by": "publisher",
"first-page": "1228",
"journal-title": "Commun. Stat. Simul. Comput.",
"key": "5381_CR37",
"unstructured": "Austin, P. C. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun. Stat. Simul. Comput. 38, 1228–1234. https://doi.org/10.1080/03610910902859574 (2009).",
"volume": "38",
"year": "2009"
}
],
"reference-count": 37,
"references-count": 37,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-025-05381-7"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Impact of early and delayed azvudine administration on COVID-19 mortality: a retrospective study",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "15"
}
