Association of nirmatrelvir/ritonavir treatment on upper respiratory SARS-CoV-2 RT-PCR negative conversion rates among high-risk patients with COVID-19
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciac600, Jul 2022
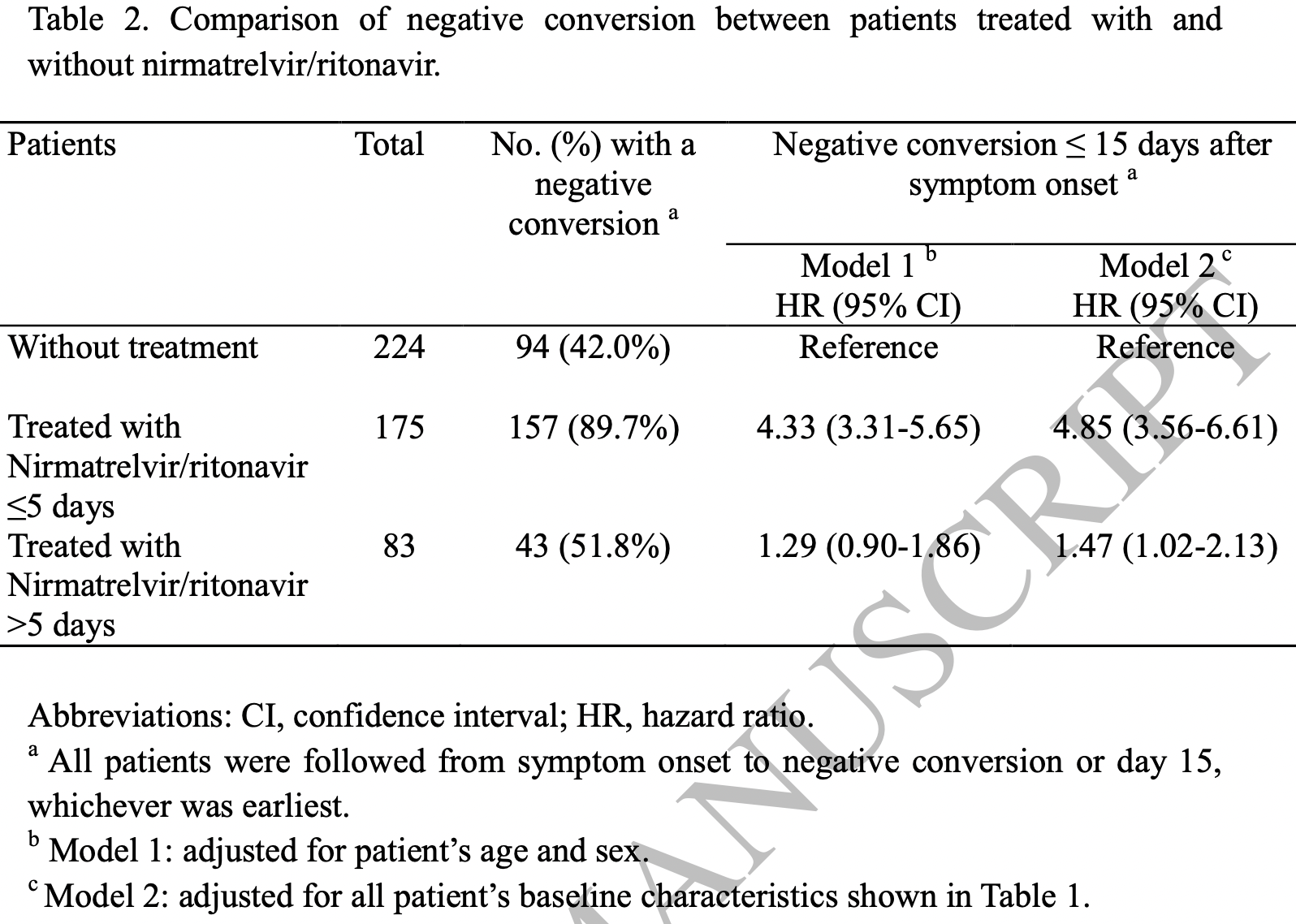
Retrospective 258 paxlovid patients and 224 patients before paxlovid was available in China, showing significantly faster viral clearance with treatment. Adjusted results are only provided for subgroups (≤5, >5 days from onset). Patients that discontinued treatment due to side effects were excluded. The treatment and control groups were separated in time. Control patients were hospitalized later, median 3 days [2-5] vs. 2 [1-3] for paxlovid patients.
Authors report some rebound events, but may not have monitored all patients for rebound. Authors report testing daily until negative conversion and it is unclear how many or when patients were tested after conversion.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments18.
|
risk of no viral clearance, 79.4% lower, HR 0.21, p < 0.001, treatment 18 of 175 (10.3%), control 130 of 224 (58.0%), NNT 2.1, adjusted per study, inverted to make HR<1 favor treatment, model 2, multivariable, day 15.
|
|
time to viral-, 41.2% lower, relative time 0.59, p < 0.001, treatment median 10.0 IQR 5.0 n=175, control median 17.0 IQR 9.0 n=224, ≤5 days, primary outcome.
|
|
risk of no viral clearance, 32.0% lower, HR 0.68, p = 0.04, treatment 40 of 83 (48.2%), control 130 of 224 (58.0%), adjusted per study, inverted to make HR<1 favor treatment, >5 days, model 2, multivariable, day 15, late treatment result.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
16.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Li et al., 23 Jul 2022, retrospective, China, peer-reviewed, 20 authors, study period 5 March, 2022 - 5 April, 2022, average treatment delay 2.0 days.
Contact: zhengyang@jlu.edu.cn.
Association of nirmatrelvir/ritonavir treatment on upper respiratory SARS-CoV-2 RT-PCR negative conversion rates among high-risk patients with COVID-19
doi:10.1093/cid/ciac600/6649232
Background: Acceleration of negative respiratory conversion of SARS-CoV-2 in patients with coronavirus disease 2019 (COVID-19) might reduce viral transmission. Nirmatrelvir/ritonavir is a new antiviral agent recently approved for treatment of COVID-19 that has the potential to facilitate negative conversion. Methods: A cohort of hospitalized adult patients with mild-to-moderate COVID-19 who had a high-risk for progression to severe disease were studied. These patients presented with COVID-19 symptoms between March 5 and April 5, 2022. The time from positive to negative upper respiratory RT-PCR conversion was assessed by Kaplan-Meier plots and Cox proportional hazards regression with the adjustment for patients baseline demographic and clinical characteristics. Results: There were 258 patients treated with nirmatrelvir/ritonavir and 224 nontreated patients who had mild-to-moderate COVID-19. The median (interquartile range) time for patients who converted from positive to negative RT-PCR was 10 Conclusion: This cohort study of high-risk patients with mild-to-moderate COVID-19 found an association between nirmatrelvir/ritonavir treatment and accelerated negative RT-PCR respiratory SARS-CoV-2 conversion that might reduce the risk of viral shedding and disease transmission.
Conflict of Interest Disclosures: None reported. Funding/Support: None.
References
Anand, Ziebuhr, Wadhwani, Mesters, Hilgenfeld, Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs, Science
Barnett, Mehrotra, Landon, Covid-19 and the upcoming financial crisis in health care, NEJM Catalyst Innovations in Care Delivery
Borio, Bright, Emanuel, A National Strategy for COVID-19 Medical Countermeasures: Vaccines and Therapeutics, JAMA
Cevik, Kuppalli, Kindrachuk, Peiris, Virology, transmission, and pathogenesis of SARS-CoV-2
Charness, Gupta, Stack, Rapid Relapse of Symptomatic Omicron SARS-CoV-2 Infection Following Early Suppression with Nirmatrelvir/Ritonavir
Chowdhury, Luhar, Khan, Choudhury, Matin et al., Longterm strategies to control COVID-19 in low and middle-income countries: an options overview of community-based, non-pharmacological interventions, European journal of epidemiology
Docherty, Harrison, Green, Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study
Drew, Donnell, Leblanc, Mcmahon, Natin, The importance of cycle threshold values in interpreting molecular tests for SARS-CoV-2, Diagnostic microbiology and infectious disease
Gandhi, Malani, Rio, COVID-19 Therapeutics for Nonhospitalized Patients, JAMA
Gottlieb, Vaca, Paredes, Early remdesivir to prevent progression to severe Covid-19 in outpatients, New England Journal of Medicine
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for highrisk, nonhospitalized adults with COVID-19, New England Journal of Medicine
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for highrisk, nonhospitalized adults with COVID-19, New England Journal of Medicine
Harrison, Lin, Wang, Mechanisms of SARS-CoV-2 transmission and pathogenesis, Trends in immunology
He, Zeng, Xiao, When and How to Adjust Non-Pharmacological Interventions Concurrent with Booster Vaccinations Against COVID-19-Guangdong, China, 2022, China CDC Weekly
Kim, Garg, 'halloran, Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET), Clinical Infectious Diseases
Kotecha, Light, Checcucci, Repurposing of drugs for Covid-19: a systematic review and meta-analysis, MedRxiv
Lamb, Nirmatrelvir Plus Ritonavir: First Approval, Drugs
Li, Wang, Lavrijsen, SARS-CoV-2 Omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination, Cell Research
Lim, Hor, Tay, Efficacy of Ivermectin treatment on disease progression among adults with mild to moderate COVID-19 and comorbidities: the I-TECH randomized clinical trial, JAMA Internal Medicine
Owen, Allerton, Anderson, An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19, Science
Ranganath, Horo, Challener, Rebound Phenomenon after Nirmatrelvir/Ritonavir Treatment of Coronavirus Disease-2019 in High-Risk Persons, Clinical Infectious Diseases
Rao, Manissero, Steele, Pareja, A systematic review of the clinical utility of cycle threshold values in the context of COVID-19, Infectious diseases and therapy
Rhee, Kanjilal, Baker, Klompas, Duration of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infectivity: when is it safe to discontinue isolation?, Clinical infectious diseases
Sevrioukova, Poulos, Structure and mechanism of the complex between cytochrome P4503A4 and ritonavir, Proceedings of the National Academy of Sciences
Singh, Toussi, Hackman, Innovative Randomized Phase 1 Study and Dosing Regimen Selection to Accelerate and Inform Pivotal COVID-19 Trial of Nirmatrelvir, Clinical Pharmacology & Therapeutics
Thakur, Dubey, Benitez, A systematic review and meta-analysis of geographic differences in comorbidities and associated severity and mortality among individuals with COVID-19, Scientific reports
Ullrich, Ekanayake, Otting, Nitsche, Main protease mutants of SARS-CoV-2 variants remain susceptible to nirmatrelvir, Bioorganic & Medicinal Chemistry Letters
Van Kampen, De Vijver, Fraaij, Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19), Nature communications
Vangeel, Chiu, Jonghe, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern, Antiviral Research
Verna, Alallon, Murakami, Analytical Performance of COVID-19 Detection Methods (RT-PCR): Scientific and Societal Concerns, Life
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention, jama
Yu, Cai, Deng, Projecting the impact of the introduction of SARS-CoV-2 Omicron variant in China in the context of waning immunity after vaccination
Zhang, Liang, Tang, Negative Conversion Rate of SARS-CoV-2
DOI record:
{
"DOI": "10.1093/cid/ciac600",
"ISSN": [
"1058-4838",
"1537-6591"
],
"URL": "http://dx.doi.org/10.1093/cid/ciac600",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Acceleration of negative respiratory conversion of SARS-CoV-2 in patients with coronavirus disease 2019 (COVID-19) might reduce viral transmission. Nirmatrelvir/ritonavir is a new antiviral agent recently approved for treatment of COVID-19 that has the potential to facilitate negative conversion.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>A cohort of hospitalized adult patients with mild-to-moderate COVID-19 who had a high-risk for progression to severe disease were studied. These patients presented with COVID-19 symptoms between March 5 and April 5, 2022. The time from positive to negative upper respiratory RT-PCR conversion was assessed by Kaplan-Meier plots and Cox proportional hazards regression with the adjustment for patients baseline demographic and clinical characteristics.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>There were 258 patients treated with nirmatrelvir/ritonavir and 224 non-treated patients who had mild-to-moderate COVID-19. The median (interquartile range) time for patients who converted from positive to negative RT-PCR was 10 days (7-12 days) in patients treated ≤5 days after symptom onset and 17 days (12-21 days) in non-treated patients, respectively. The proportions of patients with a negative conversion at day 15 were 89.7% and 42.0% in treated patients and non-treated patients, corresponding to a hazard ratio of 4.33 (95% CI, 3.31-5.65). Adjustment for baseline differences between the groups had little effect on the association. Subgroup analysis on treated patients suggests that time to negative conversion did not vary with the patients’ baseline characteristics.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>This cohort study of high-risk patients with mild-to-moderate COVID-19 found an association between nirmatrelvir/ritonavir treatment and accelerated negative RT-PCR respiratory SARS-CoV-2 conversion that might reduce the risk of viral shedding and disease transmission.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Nursing Department, The First Hospital of Jilin University , Changchun , China"
}
],
"family": "Li",
"given": "Hongyan",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Hepatobiliary and Pancreatic Surgery, The First Hospital of Jilin University , Changchun , China"
}
],
"family": "Gao",
"given": "Menghan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pediatrics, First Hospital of Jilin University , Changchun , China"
}
],
"family": "You",
"given": "Hailong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, The First Hospital of Jilin University , Changchun , China"
}
],
"family": "Zhang",
"given": "Peng",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Epidemiology, The First Hospital of Jilin University , Changchun , China"
}
],
"family": "Pan",
"given": "Yuchen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Intensive Care Unit, The First Hospital of Jilin University , Changchun , China"
}
],
"family": "Li",
"given": "Nan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Cardiovascular Medicine, The First Hospital of Jilin University , Changchun , China"
}
],
"family": "Qin",
"given": "Ling",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Endocrinology and Metabolism, The First Hospital of Jilin University , Changchun , China"
}
],
"family": "Wang",
"given": "Heyuan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory Medicine, The First Hospital of Jilin University , Changchun , China"
}
],
"family": "Li",
"given": "Dan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory Medicine, The First Hospital of Jilin University , Changchun , China"
}
],
"family": "Li",
"given": "Yang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pediatric Respiratory Medicine, The First Hospital of Jilin University , Changchun , China"
}
],
"family": "Qiao",
"given": "Hongmei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Intensive Care Unit, The First Hospital of Jilin University , Changchun , China"
}
],
"family": "Gu",
"given": "Lina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Neurosurgery, the First Hospital of Jilin University , Changchun , China"
}
],
"family": "Xu",
"given": "Songbai",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Endocrinology and Metabolism, The First Hospital of Jilin University , Changchun , China"
}
],
"family": "Guo",
"given": "Weiying",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Cancer Center, the First Hospital of Jilin University , Changchun , China"
}
],
"family": "Wang",
"given": "Nanya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory Medicine, The First Hospital of Jilin University , Changchun , China"
}
],
"family": "Liu",
"given": "Chaoying",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Hepatology, The First Hospital of Jilin University , Changchun , China"
}
],
"family": "Gao",
"given": "Pujun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Hepatology, Center of Infectious Disease and Pathogen Biology, Key laboratory of Organ Regeneration and Transplantation of the Ministry of Education, State Key Laboratory of Zoonotic Disease, The First Hospital of Jilin University , Changchun , China"
}
],
"family": "Niu",
"given": "Junqi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Neology, The First Hospital of Jilin University , Changchun , China"
}
],
"family": "Cao",
"given": "Jie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Cardiovascular Medicine, The First Hospital of Jilin University , Changchun , China"
}
],
"family": "Zheng",
"given": "Yang",
"sequence": "additional"
}
],
"container-title": "Clinical Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
7,
23
]
],
"date-time": "2022-07-23T16:38:24Z",
"timestamp": 1658594304000
},
"deposited": {
"date-parts": [
[
2022,
7,
23
]
],
"date-time": "2022-07-23T16:38:24Z",
"timestamp": 1658594304000
},
"indexed": {
"date-parts": [
[
2022,
7,
23
]
],
"date-time": "2022-07-23T17:11:32Z",
"timestamp": 1658596292324
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
7,
23
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
23
]
],
"date-time": "2022-07-23T00:00:00Z",
"timestamp": 1658534400000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciac600/45052937/ciac600.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciac600/45052937/ciac600.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022,
7,
23
]
]
},
"published-online": {
"date-parts": [
[
2022,
7,
23
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciac600/6649232"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Association of nirmatrelvir/ritonavir treatment on upper respiratory SARS-CoV-2 RT-PCR negative conversion rates among high-risk patients with COVID-19",
"type": "journal-article"
}