A Randomized, Open‐Label, Controlled Clinical Trial of Azvudine Tablets in the Treatment of Mild and Common COVID‐19, a Pilot Study
et al., Advanced Science, doi:10.1002/advs.202001435, ChiCTR2000029853, Aug 2020
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.000000017 from 39 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
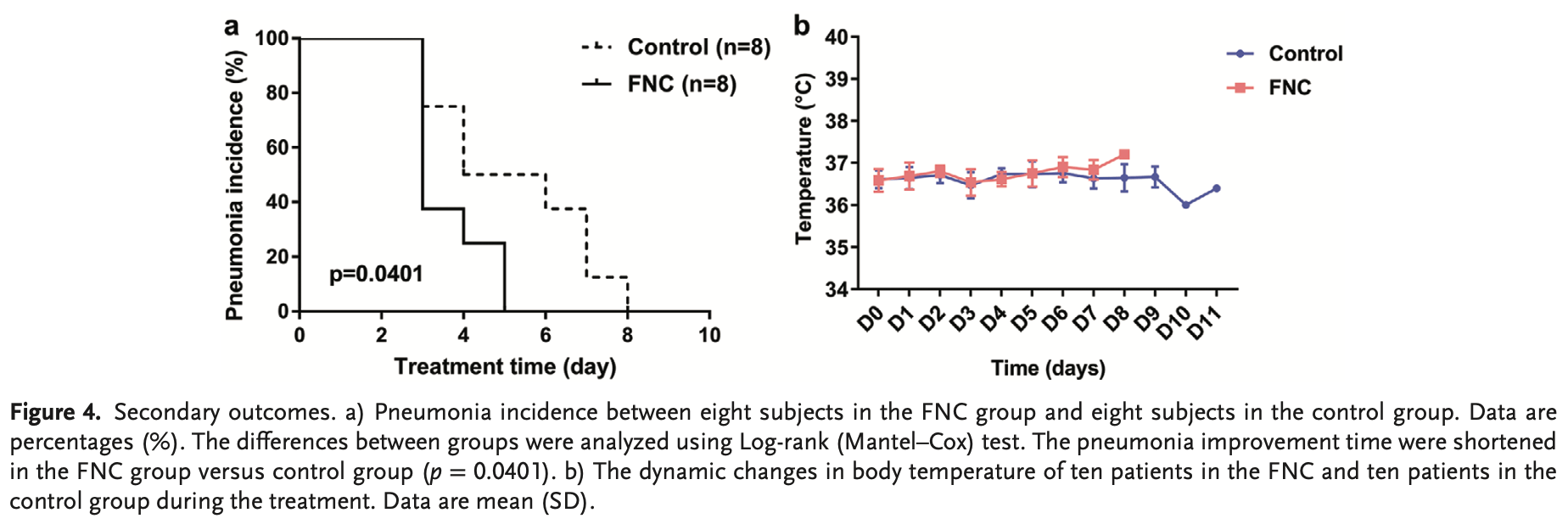
RCT 20 mild COVID-19 patients showing faster viral clearance and pneumonia improvement in chest CT images with azvudine treatment.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments3.
|
recovery time, 37.5% lower, relative time 0.62, p = 0.04, treatment 10, control 10, pneumonia resolution.
|
|
time to viral-, 53.6% lower, relative time 0.46, p = 0.008, treatment mean 2.6 (±0.97) n=10, control mean 5.6 (±3.06) n=10.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Xiong et al., Real-world data of Azvudine-induced hepatotoxicity among hospitalized COVID-19 patients in China: a retrospective case-control study, Frontiers in Pharmacology, doi:10.3389/fphar.2025.1558054.
Ren et al., 13 Aug 2020, Randomized Controlled Trial, China, peer-reviewed, median age 52.0, 22 authors, study period 18 February, 2020 - 29 February, 2020, trial ChiCTR2000029853.
Contact: fccrenzg@zzu.edu.cn, huchuansong01@163.com, changjunbiao@zzu.edu.cn, dujinfa@zsswkj.net.
A Randomized, Open‐Label, Controlled Clinical Trial of Azvudine Tablets in the Treatment of Mild and Common COVID‐19, a Pilot Study
Advanced Science, doi:10.1002/advs.202001435
Coronavirus disease 2019 (COVID-19) has spread worldwide. To date, no specific drug for COVID-19 has been developed. Thus, this randomized, open-label, controlled clinical trial (ChiCTR2000029853) was performed in China. A total of 20 mild and common COVID-19 patients were enrolled and randomly assigned to receive azvudine and symptomatic treatment (FNC group), or standard antiviral and symptomatic treatment (control group). The mean times of the first nucleic acid negative conversion (NANC) of ten patients in the FNC group and ten patients in the control group are 2.60 (SD 0.97; range 1-4) d and 5.60 (SD 3.06; range 2-13) d, respectively (p = 0.008). The mean times of the first NANC of four newly diagnosed subjects in the FNC group and ten subjects in the control group are 2.50 (SD 1.00; range 2-4) d and 9.80 (SD 4.73; range 3-19) d, respectively (starting from the initial treatment) (p = 0.01). No adverse events occur in the FNC group, while three adverse events occur in the control group (p = 0.06). The preliminary results show that FNC treatment in the mild and common COVID-19 may shorten the NANC time versus standard antiviral treatment. Therefore, clinical trials of FNC treating COVID-19 with larger sample size are warranted.
Supporting Information Supporting Information is available from the Wiley Online Library or from the author.
Conflict of Interest The authors declare no conflict of interest.
Author Contributions
References
Abreha, Hwang, Thriemer, Tadesse, Girma et al., None, PLoS Med
Azkur, Akdis, Azkur, Sokolowska, Van De Veen et al., None, Allergy
Cao, Wang, Wen, Liu, Wang et al., None, N. Engl. J. Med
Chictr, Org, A randomized, open-label, controlled clinical trial for azvudine in the treatment of novel coronavirus pneumonia (COVID-19)
Clinicaltrials, Gov, A Drug Safety and Dose-exploratory Clinical Study of Azvudine Tablets in Patients Who Have Not Received Anti-HIV Treatment (FNC)
Grein, Ohmagari, Shin, Diaz, Asperges et al., None, N. Engl. J. Med
Huang, Wang, Li, Ren, Zhao et al., None, Lancet
Jordheim, Durantel, Zoulim, Dumontet, None, Nat. Rev. Drug Discovery
Paules, Marston, Fauci, None, J. Am. Med. Assoc
Smith, Kalayanov, Sund, Winqvist, Maltseva et al., None, J. Med. Chem
Tang, Cao, Han, Wang, Chen et al., None, BMJ
Tyack, Calambokidis, Friedlaender, Goldbogen, Southall, None, PLoS One
Wang, Cao, Zhang, Yang, Liu et al., None, Cell Res
Wang, Zhang, Du, Du, Zhao et al., None, Lancet
Weiss, Leibowitz, None, Adv. Virus Res
Xu, Yang, Zheng, Zhang, Cao et al., None, J. Virol
Zhou, Zhang, Yang, Zhao, Zheng et al., None, Antiviral Ther
Zhu, Zhang, Wang, Li, Yang et al., None, N. Engl. J. Med
DOI record:
{
"DOI": "10.1002/advs.202001435",
"ISSN": [
"2198-3844",
"2198-3844"
],
"URL": "http://dx.doi.org/10.1002/advs.202001435",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Coronavirus disease 2019 (COVID‐19) has spread worldwide. To date, no specific drug for COVID‐19 has been developed. Thus, this randomized, open‐label, controlled clinical trial (ChiCTR2000029853) was performed in China. A total of 20 mild and common COVID‐19 patients were enrolled and randomly assigned to receive azvudine and symptomatic treatment (FNC group), or standard antiviral and symptomatic treatment (control group). The mean times of the first nucleic acid negative conversion (NANC) of ten patients in the FNC group and ten patients in the control group are 2.60 (SD 0.97; range 1–4) d and 5.60 (SD 3.06; range 2–13) d, respectively (<jats:italic>p</jats:italic> = 0.008). The mean times of the first NANC of four newly diagnosed subjects in the FNC group and ten subjects in the control group are 2.50 (SD 1.00; range 2–4) d and 9.80 (SD 4.73; range 3–19) d, respectively (starting from the initial treatment) (<jats:italic>p</jats:italic> = 0.01). No adverse events occur in the FNC group, while three adverse events occur in the control group (<jats:italic>p</jats:italic> = 0.06). The preliminary results show that FNC treatment in the mild and common COVID‐19 may shorten the NANC time versus standard antiviral treatment. Therefore, clinical trials of FNC treating COVID‐19 with larger sample size are warranted.</jats:p>",
"alternative-id": [
"10.1002/advs.202001435"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2020-04-19"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2020-08-13"
}
],
"author": [
{
"affiliation": [
{
"name": "Department of Infectious Diseases the First Affiliated Hospital of Zhengzhou University Zhengzhou 450052 China"
},
{
"name": "Guangshan County People's Hospital Guangshan County Xinyang 465450 China"
}
],
"family": "Ren",
"given": "Zhigang",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Guangshan County People's Hospital Guangshan County Xinyang 465450 China"
}
],
"family": "Luo",
"given": "Hong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases the First Affiliated Hospital of Zhengzhou University Zhengzhou 450052 China"
}
],
"family": "Yu",
"given": "Zujiang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Thoracic Surgery Henan Provincial Chest Hospital Zhengzhou 450008 China"
},
{
"name": "Medical Department Xixian people's Hospital Xixian Xinyang 464300 China"
}
],
"family": "Song",
"given": "Jingchao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Henan Key Laboratory of Organic Functional Molecule and Drug Innovation School of Chemistry and Chemical Engineering Henan Normal University Xinxiang 453007 China"
}
],
"family": "Liang",
"given": "Lan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Laboratory Henan Provincial Chest Hospital Zhengzhou 450008 China"
}
],
"family": "Wang",
"given": "Ling",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases the First Affiliated Hospital of Zhengzhou University Zhengzhou 450052 China"
}
],
"family": "Wang",
"given": "Haiyu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases the First Affiliated Hospital of Zhengzhou University Zhengzhou 450052 China"
}
],
"family": "Cui",
"given": "Guangying",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Henan Genuine Biotech Co., Ltd. 10 Fuxing Road, Xincheng District Pingdingshan Henan 467036 China"
}
],
"family": "Liu",
"given": "Yong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Orthopedics Huangchuan County People's Hospital Huangchuan County Xinyang 465150 China"
}
],
"family": "Wang",
"given": "Jin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medical Department Xixian people's Hospital Xixian Xinyang 464300 China"
}
],
"family": "Li",
"given": "Qingquan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Guangshan County People's Hospital Guangshan County Xinyang 465450 China"
}
],
"family": "Zeng",
"given": "Zhaohai",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Guangshan County People's Hospital Guangshan County Xinyang 465450 China"
}
],
"family": "Yang",
"given": "Shengkun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Guangshan County People's Hospital Guangshan County Xinyang 465450 China"
}
],
"family": "Pei",
"given": "Guangzhong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Guangshan County People's Hospital Guangshan County Xinyang 465450 China"
},
{
"name": "Department of Thoracic Surgery Henan Provincial Chest Hospital Zhengzhou 450008 China"
}
],
"family": "Zhu",
"given": "Yonghui",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Guangshan County People's Hospital Guangshan County Xinyang 465450 China"
}
],
"family": "Song",
"given": "Wenbin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Henan new drug creation and drug safety evaluation Collaborative Innovation Center Zhengzhou University Zhengzhou 450001 China"
}
],
"family": "Yu",
"given": "Wenquan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Henan new drug creation and drug safety evaluation Collaborative Innovation Center Zhengzhou University Zhengzhou 450001 China"
}
],
"family": "Song",
"given": "Chuanjun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Henan new drug creation and drug safety evaluation Collaborative Innovation Center Zhengzhou University Zhengzhou 450001 China"
}
],
"family": "Dong",
"given": "Lihong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Guangshan County People's Hospital Guangshan County Xinyang 465450 China"
}
],
"family": "Hu",
"given": "Chuansong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Henan Genuine Biotech Co., Ltd. 10 Fuxing Road, Xincheng District Pingdingshan Henan 467036 China"
}
],
"family": "Du",
"given": "Jinfa",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-6236-1256",
"affiliation": [
{
"name": "Henan Key Laboratory of Organic Functional Molecule and Drug Innovation School of Chemistry and Chemical Engineering Henan Normal University Xinxiang 453007 China"
},
{
"name": "Henan new drug creation and drug safety evaluation Collaborative Innovation Center Zhengzhou University Zhengzhou 450001 China"
}
],
"authenticated-orcid": false,
"family": "Chang",
"given": "Junbiao",
"sequence": "additional"
}
],
"container-title": "Advanced Science",
"container-title-short": "Advanced Science",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2020,
7,
14
]
],
"date-time": "2020-07-14T17:23:19Z",
"timestamp": 1594747399000
},
"deposited": {
"date-parts": [
[
2023,
8,
25
]
],
"date-time": "2023-08-25T19:20:37Z",
"timestamp": 1692991237000
},
"funder": [
{
"DOI": "10.13039/501100012166",
"award": [
"2018YFC2000500"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100012166",
"id-type": "DOI"
}
],
"name": "National Basic Research Program of China"
},
{
"DOI": "10.13039/501100001809",
"award": [
"U1804283"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100001809",
"id-type": "DOI"
}
],
"name": "National Natural Science Foundation of China"
}
],
"indexed": {
"date-parts": [
[
2025,
5,
19
]
],
"date-time": "2025-05-19T04:22:44Z",
"timestamp": 1747628564312,
"version": "3.40.5"
},
"is-referenced-by-count": 124,
"issue": "19",
"issued": {
"date-parts": [
[
2020,
8,
13
]
]
},
"journal-issue": {
"issue": "19",
"published-print": {
"date-parts": [
[
2020,
10
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
8,
13
]
],
"date-time": "2020-08-13T00:00:00Z",
"timestamp": 1597276800000
}
}
],
"link": [
{
"URL": "https://api.wiley.com/onlinelibrary/tdm/v1/articles/10.1002%2Fadvs.202001435",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/advs.202001435",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/advs.202001435",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/advs.202001435",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2020,
8,
13
]
]
},
"published-online": {
"date-parts": [
[
2020,
8,
13
]
]
},
"published-print": {
"date-parts": [
[
2020,
10
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1001/jama.2020.0757",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_1_1"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_1_2"
},
{
"DOI": "10.1056/NEJMoa2001017",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_1_3"
},
{
"key": "e_1_2_9_2_1",
"unstructured": "Coronavirus disease 2019 (COVID‐19): situation report‐163 1 July 2020 https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200701-covid-19-sitrep-163.pdf?sfvrsn=c202f05b_2(accessed: July2020)."
},
{
"DOI": "10.1016/B978-0-12-385885-6.00009-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_3_1"
},
{
"DOI": "10.1038/nrd4010",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_4_1"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_5_1"
},
{
"DOI": "10.1056/NEJMoa2007016",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_6_1"
},
{
"DOI": "10.1371/journal.pone.0142287",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_7_1"
},
{
"DOI": "10.1021/jm801595c",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_8_1"
},
{
"author": "Xu N.",
"first-page": "e00204",
"journal-title": "J. Virol.",
"key": "e_1_2_9_9_1",
"volume": "94",
"year": "2020"
},
{
"DOI": "10.3851/IMP2292",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_10_1"
},
{
"key": "e_1_2_9_11_1",
"unstructured": "ClinicalTrials.gov. A Drug Safety and Dose‐exploratory Clinical Study of Azvudine Tablets in Patients Who Have Not Received Anti‐HIV Treatment (FNC) https://clinicaltrials.gov/ct2/show/NCT04109183?term=AZVUDINE&draw=2&rank=2term=NCT04257656&draw=2&rank=1 (accessed: October2020)."
},
{
"key": "e_1_2_9_12_1",
"unstructured": "Chictr.org. A randomized open‐label controlled clinical trial for azvudine in the treatment of novel coronavirus pneumonia (COVID‐19) http://www.chictr.org.cn/showproj.aspx?proj=49532(accessed: February2020)."
},
{
"key": "e_1_2_9_13_1",
"unstructured": "a)Diagnosis and treatment of pneumonia infected by novel coronavirus (revised version of the fifth edition on trial) http://www.nhc.gov.cn/yzygj/s7653p/202002/d4b895337e19445f8d728fcaf1e3e13a/files/ab6bec7f93e64e7f998d802991203cd6.pdf;"
},
{
"key": "e_1_2_9_13_2",
"unstructured": "b)Diagnosis and treatment of pneumonia infected by novel coronavirus (the sixth edition on trial) http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b817.pdf"
},
{
"DOI": "10.1136/bmj.m1849",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_14_1"
},
{
"DOI": "10.1111/all.14364",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_15_1"
},
{
"DOI": "10.1371/journal.pmed.1002299",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_16_1"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_17_1"
},
{
"DOI": "10.1056/NEJMoa2001282",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_18_1"
}
],
"reference-count": 21,
"references-count": 21,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/advs.202001435"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "A Randomized, Open‐Label, Controlled Clinical Trial of Azvudine Tablets in the Treatment of Mild and Common COVID‐19, a Pilot Study",
"type": "journal-article",
"update-policy": "https://doi.org/10.1002/crossmark_policy",
"volume": "7"
}
