Oral Azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2023.101981, May 2023
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.000000017 from 39 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
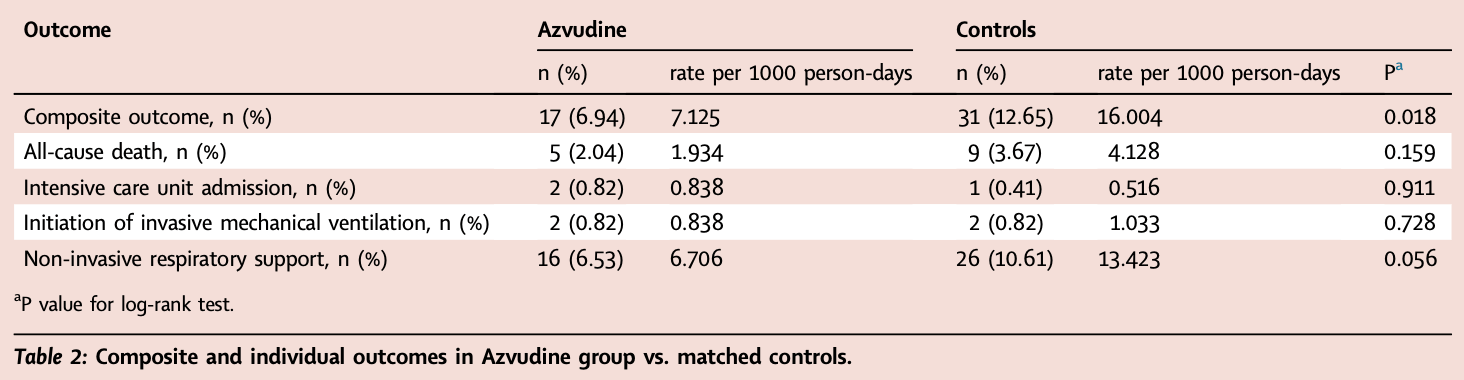
PSM retrospective 490 hospitalized COVID-19 patients with pre-existing conditions in China showing that azvudine was associated with a significantly lower risk of the composite outcome of disease progression, driven largely by lower rates of non-invasive respiratory support. However, there was no significant difference in all-cause mortality or other individual outcomes like ICU admission or invasive mechanical ventilation between the azvudine and control groups.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments3.
|
risk of death, 54.1% lower, HR 0.46, p = 0.16, treatment 5 of 245 (2.0%), control 9 of 245 (3.7%), NNT 61, odds ratio converted to relative risk, Cox proportional hazards.
|
|
risk of mechanical ventilation, no change, RR 1.00, p = 1.00, treatment 2 of 245 (0.8%), control 2 of 245 (0.8%).
|
|
risk of ICU admission, 100% higher, RR 2.00, p = 1.00, treatment 2 of 245 (0.8%), control 1 of 245 (0.4%).
|
|
risk of oxygen therapy, 38.5% lower, RR 0.62, p = 0.15, treatment 16 of 245 (6.5%), control 26 of 245 (10.6%), NNT 25.
|
|
risk of progression, 47.6% lower, HR 0.52, p = 0.02, treatment 17 of 245 (6.9%), control 31 of 245 (12.7%), NNT 18, odds ratio converted to relative risk, non-invasive respiratory support, endotracheal intubation, ICU admission, and all-cause death, Cox proportional hazards, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Xiong et al., Real-world data of Azvudine-induced hepatotoxicity among hospitalized COVID-19 patients in China: a retrospective case-control study, Frontiers in Pharmacology, doi:10.3389/fphar.2025.1558054.
Sun et al., 5 May 2023, retrospective, China, peer-reviewed, 7 authors, study period 5 December, 2022 - 31 January, 2023.
Contact: dengguangtong@outlook.com, chenxiangck@126.com, shenmx1988@csu.edu.cn.
Oral Azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study
eClinicalMedicine, doi:10.1016/j.eclinm.2023.101981
Background As the COVID-19 pandemic continues to spread, the number of associated deaths continues to increase, especially among those with pre-existing conditions. Azvudine is recommended as a priority treatment for patients with COVID-19, but its efficacy in patients with pre-existing conditions is unknown. Methods This is a single-centre, retrospective cohort study between December 5, 2022 and January 31, 2023 in Xiangya Hospital of Central South University in China to evaluate the clinical efficacy of Azvudine in hospitalised patients with COVID-19 and pre-existing conditions. Patients with Azvudine and controls were propensity score-matched (1:1) for age, gender, vaccination status, time from symptom onset to treatment exposure, severity at admission, concomitant treatments initiated at admission. The primary outcome was a composite outcome of disease progression, and the secondary outcome was each of these individual disease progression outcomes. The univariate Cox regression model was used to estimate a hazard ratio (HR) with 95% confidence interval (CI) for each result between the groups. Findings We identified 2118 hospitalised patients with COVID-19 during the study period, with a follow-up of up to 38 days. After exclusions and propensity score matching, we included 245 Azvudine recipients and 245 matched controls. Azvudine recipients had lower crude incidence rate of composite disease progression outcome compared with matched controls (7.125/1000 person-days vs. 16.004/1000 person-days, P = 0.018). There was no significant difference in all-cause death between these two groups (1.934/1000 person-days vs. 4.128/1000 person-days, P = 0.159). Azvudine treatment was associated with significantly lower risks of composite disease progression outcome compared with matched controls (HR: 0.49; 95% CI: 0.27-0.89, P = 0.016). A significant difference in all-cause death was not found (HR: 0.45; 95% CI: 0.15-1.36, P = 0.148). Interpretation These findings indicate that Azvudine therapy showed substantial clinical benefits in hospitalised patients with COVID-19 and pre-existing conditions, and should be considered for this population of patients.
Declaration of interests The authors declare no conflicts of interest that pertain to this work. Appendix A. Supplementary data Supplementary data related to this article can be found at https://doi. org/10.1016/j.eclinm.2023.101981.
References
Austin, Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations, Biom J
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med
Brown, Lang, Zhou, Why molnupiravir fails in hospitalized patients, mBio
Chen, Expert consensus on the application of Azovudine tablets in the treatment of novel coronavirus infection, Chi J China Pharm
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19, N Engl J Med
Lieber, Cox, Sourimant, SARS-CoV-2 VOC type and biological sex affect molnupiravir efficacy in severe COVID-19 dwarf hamster model, Nat Commun
Liu, Pan, Zhang, Efficacy and safety of Paxlovid in severe adult patients with SARS-Cov-2 infection: a multicenter randomized controlled study, Lancet Reg Health West Pac
Makady, De Boer, Hillege, Klungel, Goettsch, What is real-world data? A review of definitions based on literature and stakeholder interviews, Value Health
Pawlowski, Venkatakrishnan, Ramudu, Pre-existing conditions are associated with COVID-19 patients' hospitalization, despite confirmed clearance of SARS-CoV-2 virus, eClinicalMedicine
Ren, Luo, Yu, A randomized, open-label, controlled clinical trial of Azvudine tablets in the treatment of mild and common COVID-19, a pilot study, Adv Sci (Weinh)
Renoux, Azoulay, Suissa, Biases in evaluating the safety and effectiveness of drugs for the treatment of COVID-19: designing real-world evidence studies, Am J Epidemiol
Shen, Xiao, Sun, Real-world effectiveness of Azvudine in hospitalized patients with COVID-19: a retrospective cohort study, medRxiv, doi:10.1101/2023.01.23.23284899
Treskova-Schwarzbach, Haas, Reda, Pre-existing health conditions and severe COVID-19 outcomes: an umbrella review approach and meta-analysis of global evidence, BMC Med
Vogelberg, Klimek, Bruggenjurgen, Jutel, Real-world evidence for the long-term effect of allergen immunotherapy: current status on database-derived European studies, Allergy
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of early molnupiravir or nirmatrelvirritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study, Lancet Infect Dis
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study, Lancet
Yu, Chang, The first Chinese oral anti-COVID-19 drug Azvudine launched, Innovation (Camb)
Zhang, Li, Wang, Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients, Signal Transduct Target Ther
DOI record:
{
"DOI": "10.1016/j.eclinm.2023.101981",
"ISSN": [
"2589-5370"
],
"URL": "http://dx.doi.org/10.1016/j.eclinm.2023.101981",
"alternative-id": [
"S258953702300158X"
],
"article-number": "101981",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Oral Azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "eClinicalMedicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.eclinm.2023.101981"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 The Author(s). Published by Elsevier Ltd."
}
],
"author": [
{
"affiliation": [],
"family": "Sun",
"given": "Yuming",
"sequence": "first"
},
{
"affiliation": [],
"family": "Jin",
"given": "Liping",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dian",
"given": "Yating",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0441-9303",
"affiliation": [],
"authenticated-orcid": false,
"family": "Shen",
"given": "Minxue",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zeng",
"given": "Furong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Xiang",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4424-9727",
"affiliation": [],
"authenticated-orcid": false,
"family": "Deng",
"given": "Guangtong",
"sequence": "additional"
}
],
"container-title": "eClinicalMedicine",
"container-title-short": "eClinicalMedicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
5,
5
]
],
"date-time": "2023-05-05T15:38:16Z",
"timestamp": 1683301096000
},
"deposited": {
"date-parts": [
[
2023,
5,
5
]
],
"date-time": "2023-05-05T15:38:29Z",
"timestamp": 1683301109000
},
"indexed": {
"date-parts": [
[
2023,
5,
6
]
],
"date-time": "2023-05-06T04:27:48Z",
"timestamp": 1683347268100
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
5
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
5,
1
]
],
"date-time": "2023-05-01T00:00:00Z",
"timestamp": 1682899200000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
4,
13
]
],
"date-time": "2023-04-13T00:00:00Z",
"timestamp": 1681344000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S258953702300158X?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S258953702300158X?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "101981",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
5
]
]
},
"published-print": {
"date-parts": [
[
2023,
5
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/j.eclinm.2021.100793",
"article-title": "Pre-existing conditions are associated with COVID-19 patients' hospitalization, despite confirmed clearance of SARS-CoV-2 virus",
"author": "Pawlowski",
"doi-asserted-by": "crossref",
"journal-title": "eClinicalMedicine",
"key": "10.1016/j.eclinm.2023.101981_bib2",
"volume": "34",
"year": "2021"
},
{
"DOI": "10.1186/s12916-021-02058-6",
"article-title": "Pre-existing health conditions and severe COVID-19 outcomes: an umbrella review approach and meta-analysis of global evidence",
"author": "Treskova-Schwarzbach",
"doi-asserted-by": "crossref",
"first-page": "212",
"issue": "1",
"journal-title": "BMC Med",
"key": "10.1016/j.eclinm.2023.101981_bib3",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2023.101981_bib4",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2023.101981_bib5",
"volume": "386",
"year": "2022"
},
{
"article-title": "The first Chinese oral anti-COVID-19 drug Azvudine launched",
"author": "Yu",
"issue": "6",
"journal-title": "Innovation (Camb)",
"key": "10.1016/j.eclinm.2023.101981_bib6",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(22)00507-2",
"article-title": "Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study",
"author": "Wong",
"doi-asserted-by": "crossref",
"first-page": "1681",
"issue": "12",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.eclinm.2023.101981_bib11",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)01586-0",
"author": "Wong",
"doi-asserted-by": "crossref",
"first-page": "1213",
"issue": "10359",
"journal-title": "Lancet",
"key": "10.1016/j.eclinm.2023.101981_bib12",
"volume": "400",
"year": "2022"
},
{
"DOI": "10.1038/s41392-021-00835-6",
"article-title": "Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "414",
"issue": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "10.1016/j.eclinm.2023.101981_bib13",
"volume": "6",
"year": "2021"
},
{
"article-title": "A randomized, open-label, controlled clinical trial of Azvudine tablets in the treatment of mild and common COVID-19, a pilot study",
"author": "Ren",
"issue": "19",
"journal-title": "Adv Sci (Weinh)",
"key": "10.1016/j.eclinm.2023.101981_bib14",
"volume": "7",
"year": "2020"
},
{
"article-title": "Real-world effectiveness of Azvudine in hospitalized patients with COVID-19: a retrospective cohort study",
"author": "Shen",
"journal-title": "medRxiv",
"key": "10.1016/j.eclinm.2023.101981_bib15",
"year": "2023"
},
{
"DOI": "10.1093/aje/kwab028",
"article-title": "Biases in evaluating the safety and effectiveness of drugs for the treatment of COVID-19: designing real-world evidence studies",
"author": "Renoux",
"doi-asserted-by": "crossref",
"first-page": "1452",
"issue": "8",
"journal-title": "Am J Epidemiol",
"key": "10.1016/j.eclinm.2023.101981_bib18",
"volume": "190",
"year": "2021"
},
{
"DOI": "10.1002/bimj.200810488",
"article-title": "Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations",
"author": "Austin",
"doi-asserted-by": "crossref",
"first-page": "171",
"issue": "1",
"journal-title": "Biom J",
"key": "10.1016/j.eclinm.2023.101981_bib19",
"volume": "51",
"year": "2009"
},
{
"DOI": "10.1128/mbio.02916-22",
"article-title": "Why molnupiravir fails in hospitalized patients",
"author": "Brown",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "mBio",
"key": "10.1016/j.eclinm.2023.101981_bib20",
"volume": "13",
"year": "2022"
},
{
"article-title": "Efficacy and safety of Paxlovid in severe adult patients with SARS-Cov-2 infection: a multicenter randomized controlled study",
"author": "Liu",
"journal-title": "Lancet Reg Health West Pac",
"key": "10.1016/j.eclinm.2023.101981_bib21",
"volume": "33",
"year": "2023"
},
{
"DOI": "10.1038/s41467-022-32045-1",
"article-title": "SARS-CoV-2 VOC type and biological sex affect molnupiravir efficacy in severe COVID-19 dwarf hamster model",
"author": "Lieber",
"doi-asserted-by": "crossref",
"first-page": "4416",
"issue": "1",
"journal-title": "Nat Commun",
"key": "10.1016/j.eclinm.2023.101981_bib22",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1111/all.15506",
"article-title": "Real-world evidence for the long-term effect of allergen immunotherapy: current status on database-derived European studies",
"author": "Vogelberg",
"doi-asserted-by": "crossref",
"first-page": "3584",
"issue": "12",
"journal-title": "Allergy",
"key": "10.1016/j.eclinm.2023.101981_bib23",
"volume": "77",
"year": "2022"
},
{
"DOI": "10.1016/j.jval.2017.03.008",
"article-title": "What is real-world data? A review of definitions based on literature and stakeholder interviews",
"author": "Makady",
"doi-asserted-by": "crossref",
"first-page": "858",
"issue": "7",
"journal-title": "Value Health",
"key": "10.1016/j.eclinm.2023.101981_bib24",
"volume": "20",
"year": "2017"
},
{
"article-title": "Expert consensus on the application of Azovudine tablets in the treatment of novel coronavirus infection",
"author": "Chen",
"first-page": "1",
"issue": "3",
"journal-title": "Chi J China Pharm",
"key": "10.1016/j.eclinm.2023.101981_bib25",
"volume": "32",
"year": "2023"
}
],
"reference-count": 18,
"references-count": 18,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S258953702300158X"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Oral Azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "59"
}
