Low Rates of Hospitalization and Death in 4376 COVID-19 Patients Treated With Early Ambulatory Medical and Supportive Care: A Case Series and Observational Study
et al., Journal of Independent Medicine, doi:10.71189/JIM/2025/V01N02A06, Jan 2022 (preprint)
Retrospective 4,376 patients with mild/moderate COVID-19 in the USA treated with multiple medications including HCQ/ivermectin, zinc, azithromycin, budesonide, and dexamethasone (exact treatments specific to each patient), showing significantly lower hospitalization and mortality compared to the surrounding community.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
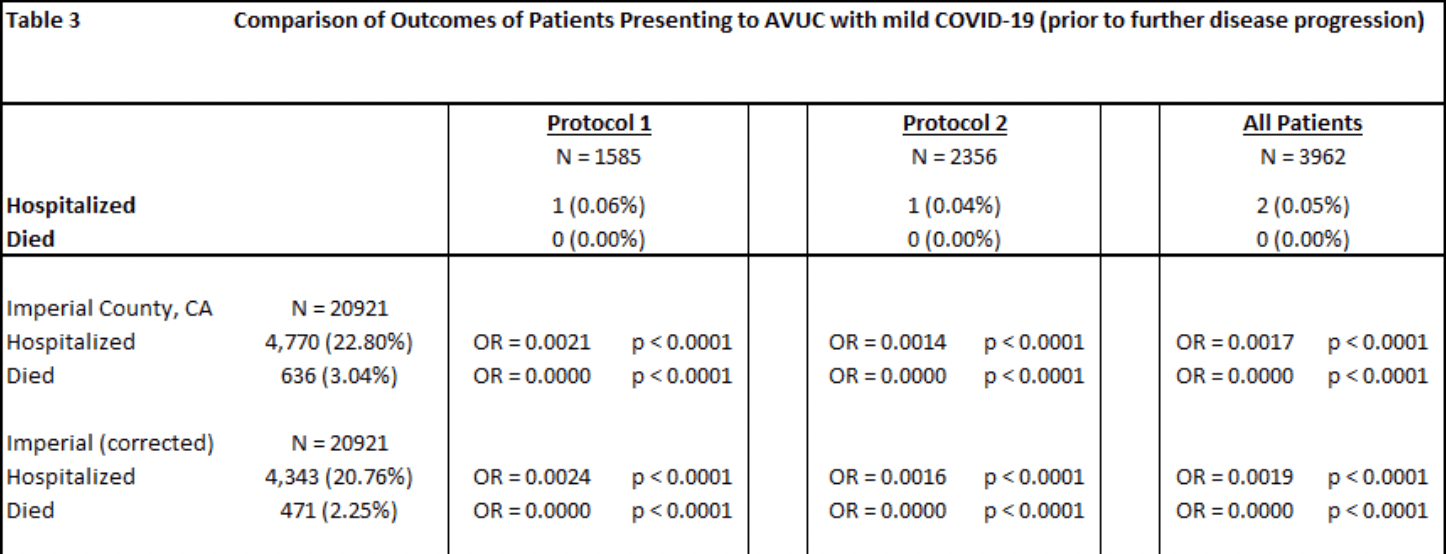
risk of death, 99.9% lower, RR 0.001, p < 0.001, treatment 0 of 3,962 (0.0%), control 636 of 20,921 (3.0%), NNT 33, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), All AVUC mild patients vs. Imperial County (corrected).
|
|
risk of hospitalization, 99.8% lower, RR 0.002, p < 0.001, treatment 2 of 3,962 (0.1%), control 4,770 of 20,921 (22.8%), NNT 4.4, All AVUC mild patients vs. Imperial County (corrected).
|
|
risk of death, 97.7% lower, RR 0.02, p < 0.001, treatment 3 of 4,374 (0.1%), control 636 of 20,921 (3.0%), NNT 34, All AVUC patients vs. Imperial County (corrected).
|
|
risk of hospitalization, 99.1% lower, RR 0.009, p < 0.001, treatment 9 of 4,374 (0.2%), control 4,770 of 20,921 (22.8%), NNT 4.4, All AVUC patients vs. Imperial County (corrected).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Tyson et al., 13 Jan 2022, retrospective, USA, peer-reviewed, 13 authors, this trial uses multiple treatments in the treatment arm (combined with multiple treatments) - results of individual treatments may vary.
Contact: btysonmd@gmail.com.
Low Rates of Hospitalization and Death in 4376 COVID-19 Patients Treated With Early Ambulatory Medical and Supportive Care: A Case Series and Observational Study
Journal of Independent Medicine, doi:10.71189/jim/2025/v01n02a06
This study evaluates early ambulatory protocols for treating 4376 COVID-19 patients at All Valley Urgent Care (AVUC) facilities in Imperial County, California, and compares outcomes with other patients in the same region during a nearly identical period. The goal was to contribute to evidence on whether early outpatient treatment reduces hospitalization and mortality rates. The protocols, based on data from neighboring countries, included Protocol 1 (a multivitamin pack, selective use of hydroxychloroquine, two antibiotics, and inhaled steroids) and Protocol 2 (which added ivermectin). Results were stratified by disease severity at presentation. The average patient age was 40.5 years; 12.8% of patients were under 20 years old. For the 3962 . mild COVID-19 patients treated early, no deaths occurred, compared to a 3.03% mortality rate (2.25% risk-adjusted) in the same county during the same period. Hospitalization rates for this group were 0.05%, compared to 22.68% (20.76% risk-adjusted) in the general population. When treated within 7 days, patients had a 100% success rate, while those treated later had a 99.9% success rate. Mild symptom patients had lower hospitalization (OR = 0.0293; P < .0001) and mortality (OR = 0.0000; P = .0008) rates. These results suggest the multidrug protocols significantly reduced adverse outcomes, with no serious side effects observed during followup (3-14 days).
Competing Interests None of the authors report any conflicts of interest.
Authors' Contribution: All authors had access to the data and wrote the manuscript. This page is intentionally left blank https://doi.org/10.71189/JIM/2025/V01N02A06
References
Berardi, The Italian doctor flattening the curve by treating COVID-19 patients in their homes, TIME
Bhattacharya, Chowdhury, Nandi, Pre-exposure hydroxychloroquine prophylaxis for COVID-19 in healthcare workers: a retrospective cohort, Int J Res Med Sci, doi:10.18203/2320-6012.ijrms20205444
Chatterjee, Anand, Singh, Healthcare workers & SARS-CoV-2 infection in India: a case-control investigation in the time of COVID-19, Indian J Med Res, doi:10.4103/ijmr.IJMR_2234_20
Colbert, Venegas-Vera, Lerma, Utility of telemedicine in the COVID-19 era, Rev Cardiovasc Med, doi:10.31083/j.rcm.2020.04.188
Colson, Rolain, Lagier, Brouqui, Raoult, Chloroquine and hydroxychloroquine as available weapons to fight COVID-19, Int J An, doi:10.71189/JIM/2025/V01N02A06
Concato, Shah, Horwitz, Randomized, controlled trials, observational studies, and the hierarchy of research designs, N Engl J Med, doi:10.1056/NEJM200006223422507
Consortium, Repurposed antiviral drugs for COVID-19-Interim WHO Solidarity trial results, N Engl J Med, doi:10.1056/NEJMoa2023184
Derwand, Scholz, Zelenko, COVID-19 outpatients: early risk-stratified treatment with zinc plus low-dose hydroxychloroquine and azithromycin: a retrospective case series study, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2020.106214
Flannery, Adkins, Cook, Unpeeling the evidence for the banana bag: evidencebased recommendations for the management of alcohol-associated vitamin and electrolyte deficiencies in the ICU, Crit Care Med, doi:10.1097/CCM.000000000000165
Gao, Tian, Yang, Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies, Biosci Trends, doi:10.5582/bst.2020.01047
Gautret, Lagier, Parola, Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2020.105949
Group, Effect of hydroxychloroquine in hospitalized patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2022926
Guérin, Lévy, Thomas, Azithromycin and hydroxychloroquine accelerate recovery of outpatients with mild/moderate COVID-19, Asian J Med Health, doi:10.20944/preprints202005.0486.v1
Hcqmeta, HCQ for COVID-19: real-time meta-analysis of 273 studies
Ip, Ahn, Zhou, Hydroxychloroquine in the treatment of outpatients with mildly symptomatic COVID-19: a multi-center observational study, BMC Infect Dis, doi:10.1186/s12879-021-05773-w
Keyaerts, Vijgen, Maes, Neyts, Van Ranst, In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine, Biochem Biophys Res Commun, doi:10.1016/j.bbrc.2004.08.085
Kory, Meduri, Varon, Iglesias, Marik, Review of the emerging evidence demonstrating the efficacy of ivermectin in the prophylaxis and treatment of COVID-19, Am J Ther, doi:10.1097/MJT.0000000000001377
Krauss, Why all randomised controlled trials produce biased results, Ann Med, doi:10.71189/JIM/2025/V01N02A06
Mccullough, Eidt, Rangaswami, Urgent need for individual mobile phone and institutional reporting of at home, hospitalized, and intensive care unit cases of SARS-CoV-2 (COVID-19) infection, Rev Cardiovasc Med, doi:10.31083/j.rcm.2020.01.42
Mccullough, Innovative early sequenced multidrug therapy for SARS-CoV-2 (COVID-19) infection to reduce hospitalization and death, Int J Med Sci Clin Invention, doi:10.31083/j.rcm.2020.04.264
Mccullough, Oskoui, Early multidrug regimens in new potentially fatal medical problems, Rev Cardiovasc Med, doi:10.31083/j.rcm.2020.04.270
Pang, Wang, Ang, Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019-nCoV): a systematic review, J Clin Med, doi:10.3390/jcm9030623
Procter, Ross, Pickard, Smith, Hanson et al., Clinical outcomes after early ambulatory multidrug therapy for highrisk SARS-CoV-2 (COVID-19) infection, Rev Cardiovasc Med, doi:10.31083/j.rcm.2020.04.260
Risch, Early outpatient treatment of symptomatic, high-risk COVID-19 patients that should be ramped up immediately as key to the pandemic crisis, Am J Epidemiol, doi:10.1093/aje/kwaa093
Rolain, Colson, Raoult, Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2007.05.015
Savarino, Boelaert, Cassone, Majori, Cauda, Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet, Infect Dis, doi:10.1016/s1473-3099(03)00806-5
Vetter, Eckerle, Kaiser, COVID-19: a puzzle with many missing pieces, BMJ, doi:10.1136/bmj.m627
Vincent, Bergeron, Benjannet, Chloroquine is a potent inhibitor of SARS coronavirus infection and spread, Virol J, doi:10.1186/1743-422X-2-69
DOI record:
{
"DOI": "10.71189/jim/2025/v01n02a06",
"ISSN": [
"3066-2354"
],
"URL": "http://dx.doi.org/10.71189/JIM/2025/V01N02A06",
"abstract": "<jats:p>This study evaluates early ambulatory protocols for treating 4376 COVID-19 patients at All Valley Urgent Care (AVUC) facilities in Imperial County, California, and compares outcomes with other patients in the same region during a nearly identical period. The goal was to contribute to evidence on whether early outpatient treatment reduces hospitalization and mortality rates. The protocols, based on data from neighboring countries, included Protocol 1 (a multivitamin pack, selective use of hydroxychloroquine, two antibiotics, and inhaled steroids) and Protocol 2 (which added ivermectin). Results were stratified by disease severity at presentation. The average patient age was 40.5 years; 12.8% of patients were under 20 years old. For the 3962 mild COVID-19 patients treated early, no deaths occurred, compared to a 3.03% mortality rate (2.25% risk-adjusted) in the same county during the same period. Hospitalization rates for this group were 0.05%, compared to 22.68% (20.76% risk-adjusted) in the general population. When treated within 7 days, patients had a 100% success rate, while those treated later had a 99.9% success rate. Mild symptom patients had lower hospitalization (OR = 0.0293; P < .0001) and mortality (OR = 0.0000; P = .0008) rates. These results suggest the multidrug protocols significantly reduced adverse outcomes, with no serious side effects observed during follow-up (3–14 days).\n\nKeywords: COVID-19, hospitalization, hydroxychloroquine, ivermectin, mortality, multidrug, SARS-CoV-2</jats:p>",
"author": [
{
"affiliation": [],
"family": "Tyson",
"given": "Brian",
"sequence": "first"
},
{
"affiliation": [],
"family": "Fareed",
"given": "George",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gutierrez",
"given": "Emmanuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Villegas",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gomez",
"given": "Edgar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lopez",
"given": "Paloma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Herrera",
"given": "Ernesto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Castro",
"given": "Miriam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Palomera III",
"given": "Jesus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morales",
"given": "Christiany",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gonzalez",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tyson",
"given": "Fabiola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Crawford",
"given": "Mathew",
"sequence": "additional"
}
],
"container-title": "Journal of Independent Medicine",
"container-title-short": "J Indep Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
4,
29
]
],
"date-time": "2025-04-29T18:07:23Z",
"timestamp": 1745950043000
},
"deposited": {
"date-parts": [
[
2025,
4,
29
]
],
"date-time": "2025-04-29T18:07:28Z",
"timestamp": 1745950048000
},
"indexed": {
"date-parts": [
[
2025,
4,
30
]
],
"date-time": "2025-04-30T04:21:05Z",
"timestamp": 1745986865317,
"version": "3.40.4"
},
"is-referenced-by-count": 0,
"issue": "02",
"issued": {
"date-parts": [
[
2025,
5,
7
]
]
},
"journal-issue": {
"issue": "02",
"published-online": {
"date-parts": [
[
2025,
5,
7
]
]
}
},
"member": "52103",
"original-title": [],
"page": "129-142",
"prefix": "10.71189",
"published": {
"date-parts": [
[
2025,
5,
7
]
]
},
"published-online": {
"date-parts": [
[
2025,
5,
7
]
]
},
"publisher": "Independent Medical Alliance",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://journalofindependentmedicine.org/articles/v01n02a06/"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Low Rates of Hospitalization and Death in 4376 COVID-19 Patients Treated With Early Ambulatory Medical and Supportive Care: A Case Series and Observational Study",
"type": "journal-article",
"volume": "01"
}
tyson