A Randomised, Double-Blind, Placebo-Controlled Trial Evaluating Concentrated Phytochemical-Rich Nutritional Capsule in Addition to a Probiotic Capsule on Clinical Outcomes among Individuals with COVID-19—The UK Phyto-V Study
et al., COVID, doi:10.3390/covid2040031, Phyto-V, Mar 2022
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 147 long COVID patients in the UK, 56 treated with a phytochemical-rich concentrated food capsule, showing improved recovery with treatment. Treatment included curcumin, bioflavonoids, chamomile, ellagic acid, and resveratrol.
This is the 10th of 21 COVID-19 RCTs for curcumin, which collectively show efficacy with p=0.0000022.
This is the 15th of 28 COVID-19 controlled studies for curcumin, which collectively show efficacy with p=0.0000000061.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments1.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
Study covers resveratrol and curcumin.
|
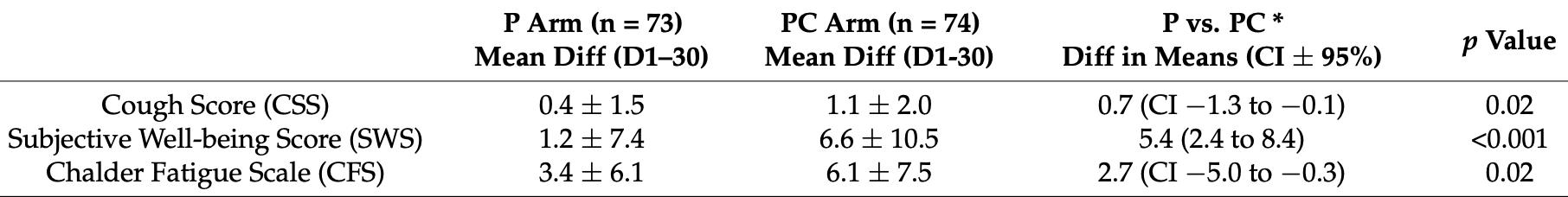
relative improvement, 44.3% better, RR 0.56, p = 0.02, treatment mean 6.1 (±7.5) n=74, control mean 3.4 (±6.1) n=73, CFS.
|
|
relative improvement, 81.8% better, RR 0.18, p < 0.001, treatment mean 6.6 (±10.5) n=74, control mean 1.2 (±7.4) n=73, SWS.
|
|
relative improvement, 63.6% better, RR 0.36, p = 0.02, treatment mean 1.1 (±2.0) n=74, control mean 0.4 (±1.5) n=73, CSS.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Thomas et al., 22 Mar 2022, Double Blind Randomized Controlled Trial, placebo-controlled, United Kingdom, peer-reviewed, 7 authors, study period May 2020 - May 2021, this trial uses multiple treatments in the treatment arm (combined with bioflavonoids, chamomile, ellagic acid, resveratrol) - results of individual treatments may vary, Phyto-V trial.
Contact: robert.thomas@bedfordhospital.nhs.uk (corresponding author), madeleine.williams@bedfordhospital.nhs.uk, yuuki.yanagisawa@bedfordhospital.nhs.uk, rajeev.kumar@bedordhospital.nhs.uk, jeffrey.aldous@beds.ac.uk, angel.chater@beds.ac.uk, rf451@cam.ac.uk.
A Randomised, Double-Blind, Placebo-Controlled Trial Evaluating Concentrated Phytochemical-Rich Nutritional Capsule in Addition to a Probiotic Capsule on Clinical Outcomes among Individuals with COVID-19—The UK Phyto-V Study
COVID, doi:10.3390/covid2040031
Gut microflora dysbiosis affects the majority of individuals after COVID-19, contributing to both gastro-intestinal (GI) and non-GI symptoms. Natural phytochemicals have reported anti-viral properties and favourable effects on inflammatory and oxidative pathways, both important for tissue damage post-viral pneumonia. This study involved 147 participants with symptomatic COVID-19, randomised to receive a placebo (P) or a phytochemical-rich concentrated food capsule (PC) in addition to a pre/probiotic lactobacillus capsule. Participants taking the PC had an almost two-fold reduction in mean fatigue scores compared to P [p = 0.02], a three-fold reduction in cough score and more than a double improvement in overall well-being scores [p = 0.02]. Two (1.5%) participants reported mild, increased bloating which they felt was attributable to the capsules, although GI symptoms improved in 25 of 31 participants (82%) who reported them at baseline. Sedentary, older, previously hospitalised men with GI symptoms had a statistically significantly improvement among those given the probiotic. Although some participants with early disease would have improved spontaneously, such a rapid improvement observed in the majority of participants, who had been suffering for an average of 108 days, was clinically relevant and welcomed, especially among those more likely to have pre-existing gut dysbiosis. We are now evaluating whether this blend could also enhance antibody titres post-COVID-19 vaccination.
Author Contributions: Conceptualization, R.T., R.K., J.A. and A.C.; methodology, R.T., J.A., A.C. and M.W.; software, Not applicable; validation, R.T. and J.A.; formal analysis, J.A.; investigation, R.T. and M.W.; resources, R.T. and M.W.; data, M.W. and R.T.; writing-original draft preparation, R.T.; writing-review and editing, Y.Y., R.F. and M.W.; visualization, M.W. and Y.Y.; supervision, R.T.; project administration, M.W.; funding acquisition, Not applicable. All authors have read and agreed to the published version of the manuscript.
References
Adnan, Dewi, Potential Effects Immunomodulators on Probiotics in COVID-19 Preventing Infection in the Future. A Narrative Review, Int. J. Med. Stud, doi:10.5195/ijms.2020.486
Al Azzaz, Al Tarraf, Heumann, Da Silva Barreira, Laurent et al., Resveratrol Favors Adhesion and Biofilm Formation of Lacticaseibacillus paracasei subsp. paracasei Strain ATCC334, Int. J. Mol. Sci, doi:10.3390/ijms21155423
Al-Howiriny, Alsheikh, Alqasoumi, Al-Yahya, Eltahir et al., Protective Effect of Origanum majorana L. 'Marjoram' on various models of gastric mucosal injury in rats, Am. J. Chin. Med, doi:10.1142/S0192415X0900703X
Alfaleh, Anabrees, Probiotics for prevention of necrotizing enterocolitis in preterm infants, Cochrane Database Syst. Rev
Alves-Santos, Sugizaki, Lima, Naves, Prebiotic effect of dietary polyphenols: A systematic review, J. Funct. Foods, doi:10.1016/j.jff.2020.104169
Alzaabi, Hamdy, Ashmawy, Hamoda, Alkhayat et al., Flavonoids are promising safe therapy against COVID-19, Phytochem. Rev. Proc. Phytochem. Soc. Eur, doi:10.1007/s11101-021-09759-z
Anand, Mande, Diet, Microbiota and Gut-Lung Connection, Front. Microbiol, doi:10.3389/fmicb.2018.02147
Arcanjo, Andrade, Padilla, Rodríguez, Madruga et al., Resveratrol protects Lactobacillus reuteri against H2O2-induced oxidative stress and stimulates antioxidant defenses through upregulation of the dhaT gene, Free. Radic. Biol. Med, doi:10.1016/j.freeradbiomed.2019.02.023
Avery, Kaiser, Sharman, Scheett, Barnes et al., Effects of vitamin E supplementation on recovery from repeated bouts of resistance exercise, J. Strength Cond. Res
Azad, Sarker, Wan, Immunomodulatory Effects of Probiotics on Cytokine Profiles, BioMed Res. Int, doi:10.1155/2018/8063647
Bailey, Diotallevi, Nicol, Mcneill, Shaw et al., Nitric Oxide Modulates Metabolic Remodeling in Inflammatory Macrophages through TCA Cycle Regulation and Itaconate Accumulation, Cell Rep, doi:10.1016/j.celrep.2019.06.018
Baud, Agri, Gibson, Reid, Giannoni, Using Probiotics to Flatten the Curve of Coronavirus Disease COVID-2019 Pandemic, Front. Public Health, doi:10.3389/fpubh.2020.00186
Beltrán-García, Osca-Verdegal, Pallardó, Ferreres, Rodríguez et al., Oxidative Stress and Inflammation in COVID-19-Associated Sepsis: The Potential Role of Anti-Oxidant Therapy in Avoiding Disease Progression, Antioxidants, doi:10.3390/antiox9100936
Bernardeau, Guguen, Vernoux, Beneficial lactobacilli in food and feed: Long-term use, biodiversity and proposals for specific and realistic safety assessments, FEMS Microbiol. Rev, doi:10.1111/j.1574-6976.2006.00020.x
Bernardeau, Vernoux, Henri-Dubernet, Guéguen, Safety assessment of dairy microorganisms: The Lactobacillus genus, Int. J. Food Microbiol, doi:10.1016/j.ijfoodmicro.2007.08.015
Biancatelli, Berrill, Catravas, Marik, Quercetin et al., An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19), Front. Immunol, doi:10.3389/fimmu.2020.01451
Bolte, Vich, Vila, Imhann, Collij et al., Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome, Gut, doi:10.1136/gutjnl-2020-322670
Borriello, Hammes, Holzapfel, Marteau, Schrezenmeir et al., Safety of probiotics that contain lactobacilli or bifidobacteria, Clin. Infect. Dis, doi:10.1086/368080
Borruel, Carol, Casellas, Antolin, De Lara et al., Increased mucosal tumour necrosis factor alpha production in Crohn's disease can be downregulated ex vivo by probiotic bacteria, Gut, doi:10.1136/gut.51.5.659
Brito, Diaz, Muñoz-Quezada, Llorente, Gil, Probiotic mechanisms of action, Ann. Nutr. Metab, doi:10.1159/000342079
Budden, Gellatly, Wood, Cooper, Morrison et al., Emerging pathogenic links between microbiota and the gut-lung axis, Nat. Rev. Microbiol, doi:10.1038/nrmicro.2016.142
Bwire, Coronavirus: Why Men are More Vulnerable to Covid-19 Than Women? SN, Compr. Clin. Med
Campagna, Rivas, Antiviral Activity of Resveratrol, Biochem. Soc. Trans, doi:10.1042/BST0380050
Carlson, Erickson, Lloyd, Slavin, Health Effects and Sources of Prebiotic Dietary Fibre, Curr. Dev. Nutr, doi:10.1093/cdn/nzy005
Cecchini, Cecchini, SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression, Med. Hypotheses, doi:10.1016/j.mehy.2020.110102
Chalder, Berelowitz, Pawlikowska, Watts, Wessely et al., Development of a fatigue scale, J. Psychosom. Res, doi:10.1016/0022-3999(93)90081-P
Chang, Ng, Sun, Lactoferrin as potential preventative and adjunct treatment for COVID-19, Int. J. Antimicrob. Agents, doi:10.1016/j.ijantimicag.2020.106118
Chen, Gu, Chen, Lu, Shi et al., Six-month follow-up of gut microbiota richness in patients with COVID-19, Gut, doi:10.1136/gutjnl-2021-324090
Conlon, Bird, The Impact of Diet and Lifestyle on Gut Microbiota and Human Health, Nutrients, doi:10.3390/nu7010017
Critchfield, Butera, Folks, Inhibition of HIV Activation in Latently Infected Cells by Flavonoid Compounds, AIDS Res. Hum. Retrovir, doi:10.1089/aid.1996.12.39
Crow, Dichlorodihydrofluorescein and dihydrorhodamine 123 are sensitive indicators of peroxynitrite in vitro: Implications for intracellular measurement of reactive nitrogen and oxygen species, Nitric Oxide, doi:10.1006/niox.1996.0113
Daneshkhah, Agrawal, Eshein, Subramanian, Roy et al., The possible role of vitamin D in suppressing cytokine storm and associated mortality in COVID-19 patients, MedRxiv
Dantzer, Heijnen, Kavelaars, Laye, Capuron, The neuroimmune basis of fatigue, Trends Neurosci
David, Maurice, Carmody, Gootenberg, Button et al., Diet rapidly and reproducibly alters the human gut microbiome, Nature, doi:10.1038/nature12820
Dehghan, Gargari, Aliasgharzadeh, Inulin controls inflammation and metabolic endotoxemia in women with type 2 diabetes mellitus: A randomized-controlled clinical trial, Int. J. Food Sci. Nutr, doi:10.3109/09637486.2013.836738
Demir, Demir, Aygun, Vitamin D deficiency is associated with COVID-19 positivity and severity of the disease, J. Med. Virol, doi:10.1002/jmv.26832
Dhar, Mohanty, Gut microbiota and Covid-19-possible link and implications, Virus Res, doi:10.1016/j.virusres.2020.198018
Dickson, The microbiome and critical illness, Lancet Respir. Med, doi:10.1016/S2213-2600(15)00427-0
Doron, Snydman, Risk and safety of probiotics, Clin. Infect. Dis, doi:10.1093/cid/civ085
Dumas, Bernard, Poquet, Lugo-Villarino, Neyrolles, The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases, Cell. Microbiol, doi:10.1111/cmi.12966
Eguchi, Fujitani, Nakagawa, Miyazaki, Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus, Sci. Rep, doi:10.1038/s41598-019-39602-7
Enaud, Prevel, Ciarlo, Beaufils, Wieërs et al., The gut-lung axis in health and respiratory diseases: A place for inter-organ and inter-kingdom crosstalks, Front. Cell. Infect. Microbiol, doi:10.3389/fcimb.2020.00009
Fanos, Pintus, Pintus, Marcialis, Lung microbiota in the acute respiratory disease: From coronavirus to metabolomics, J. Paediatr. Neonatal Individ. Med
Filardo, Di Pietro, Mastromarino, Sessa, Therapeutic Potential of Resveratrol Against Emerging Respiratory Viral Infections, Pharmacol. Ther, doi:10.1016/j.pharmthera.2020.107613
Fujita, Iimuro, Shinozaki, Sakamaki, Uemura et al., Decreased duration of acute upper respiratory tract infections with daily intake of fermented milk: A multicenter, double-blinded, randomized comparative study in users of day care facilities for the elderly population, Am. J. Infect. Control, doi:10.1016/j.ajic.2013.04.005
Funk, Oyarzo, Frye, Chen, Lantz et al., Turmeric extracts containing curcuminoids prevent experimental rheumatoid arthritis, J. Nat. Prod, doi:10.1021/np050327j
Gagliardi, Totino, Cacciotti, Lebba, Neroni et al., Rebuilding the Gut Microbiota Ecosystem, Int. J. Environ. Res. Public Health, doi:10.3390/ijerph15081679
Galland, The gut microbiome and the brain, J. Med. Food, doi:10.1089/jmf.2014.7000
Gill, Rutherfurd, Cross, Dietary probiotic supplementation enhances natural killer cell activity in the elderly: An investigation of age-related immunological changes, J. Clin. Immunol, doi:10.1023/A:1010979225018
Giloteaux, Goodrich, Walters, Levine, Ley et al., R Reduced diversity and altered composition of the gut microbiome in individuals with myalgic encephalomyelitis/chronic fatigue syndrome, Microbiome
Green, Dawson, Groenewoud, Jones, Thijssen, Is Flow-Mediated Dilation Nitric Oxide Mediated?, Hypertension, doi:10.1161/HYPERTENSIONAHA.113.02044
Gross, Jacobs, Peters, Possemiers, Van Duynhoven et al., In vitro bioconversion of polyphenols from black tea and red wine/grape juice by human intestinal microbiota displays strong inter-individual variability, J. Agric. Food Chem, doi:10.1021/jf101475m
Gu, Chen, Wu, Chen, Gao et al., Alterations of the Gut Microbiota in Patients with Coronavirus Disease or H1N1 Influenza, Clin. Infect. Dis, doi:10.1093/cid/ciaa709
Gurung, Li, You, Rodrigues, Jump et al., Role of gut microbiota in type 2 diabetes pathophysiology, EBioMedicine, doi:10.1016/j.ebiom.2019.11.051
Gutiérrez-Castrellón, Gandara-Martí, Abreu, Nieto-Rufino, López-Orduña et al., Probiotic improves symptomatic and viral clearance in Covid19 outpatients: A randomized, quadruple-blinded, placebo-controlled trial, Gut Microbes, doi:10.1080/19490976.2021.2018899
Hassan, Wahba, El-Masry, Elhamid, Boseila et al., Eating Habits and Lifestyles among a Sample of Obese Working Egyptian Women. Open Access Maced, J. Med. Sci, doi:10.3889/oamjms.2015.005
Hempel, Newberry, Ruelaz, Wang, Miles et al., Safety of probiotics used to reduce risk and prevent or treat disease, Evid. Rep. Technol. Assess
Hiippala, Jouhten, Ronkainen, Hartikainen, Kainulainen et al., The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation, Nutrients, doi:10.3390/nu10080988
Horvat, Avbelj, Duran-Alonso, Banjanac, Petkovic et al., Antiviral Activities of Halogenated Emodin Derivatives against Human Coronavirus NL63, Molecules, doi:10.3390/molecules26226825
Huang, Huang, Wang, Li, Ren et al., 6-Month consequences of COVID-19 in patients discharged from Hospital: A cohort study, Lancet, doi:10.1016/S0140-6736(20)32656-8
Jamilian, Amirani, Asemi, The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: A randomized, double-blind, placebo-controlled trial, Clin. Nutr, doi:10.1016/j.clnu.2018.10.028
Jassim, Naji, Novel antiviral agents: A medicinal plant perspective, J. Appl. Microbiol, doi:10.1046/j.1365-2672.2003.02026.x
Jennings, Parks, Curcumin as an Antiviral Agent, Viruses, doi:10.3390/v12111242
Jiang, Li, Huang, Liu, Zhao, The gut microbiota and Alzheimer's disease, J. Alzheimers Dis
Jones, Martoni, Prakash, Oral supplementation with probiotic L. reuteri increases mean circulating 25-hydroxyvitamin D: A post hoc analysis of a randomized controlled trial, J. Clin. Endocrinol. Metab, doi:10.1210/jc.2012-4262
Kang, Kim, Hwang, Ji, The effect of probiotics on prevention of common cold: A meta-analysis of randomized controlled trial studies, Korean J. Fam. Med, doi:10.4082/kjfm.2013.34.1.2
Karunaweera, Raju, Gyengesi, Munch, Plant polyphenols as inhibitors of NF-κB induced cytokine production-A potential anti-inflammatory treatment for Alzheimer's disease? Front, Mol. Neurosci, doi:10.3389/fnmol.2015.00024
Kassaa, Hober, Hamze, Chihib, Drider, Antiviral potential of lactic acid bacteria and their bacteriocins, Probiotics Antimicrob. Proteins, doi:10.1007/s12602-014-9162-6
Koh, De Vadder, Kovatcheva-Datchary, Backhed, From dietary fibre to host physiology: Short-chain fatty acids as key bacterial metabolites, Cell, doi:10.1016/j.cell.2016.05.041
Kotwal, Natural Antivirals against Human Viruses, Virol. Mycol, doi:10.4172/2161-0517.1000e107
Kumar, Basu, Rout, Manna, Dandapat et al., Curcumin and Ellagic acid synergistically induce ROS generation, DNA damage, p53 accumulation and apoptosis in HeLa cervical carcinoma cells, Biomed. Pharmacol, doi:10.1016/j.biopha.2016.03.037
Kurian, Unnikrishnan, Miraj, Bagchi, Banerjee et al., Probiotics in Prevention and Treatment of COVID-19: Current Perspective and Future Prospects, Arch. Med. Res, doi:10.1016/j.arcmed.2021.03.002
Lee, Kim, Lee, A New Anti-HIV Flavonoid Glucuronide from Chrysanthemum Morifolium, Planta Medica
Li, Chen, Zhang, Guo, Wang et al., Identification of natural compounds with antiviral activities against SARS-associated coronavirus, Antivir. Res, doi:10.1016/j.antiviral.2005.02.007
Li, Yang, Lai, Huang, Liao et al., Antiviral activity of aloe-emodin against Influenza A virus via galectin-3 up-regulation, Eur. J. Pharmacol, doi:10.1016/j.ejphar.2014.05.028
Lin, Jiang, Zhang, Huang, Zhang et al., Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection, Gut, doi:10.1136/gutjnl-2020-321013
Lin, Tsai, Tsai, Lai, Ho et al., Anti-SARS coronavirus 3C-like protease effects of plant-derived phenolic compounds, Antivir. Res, doi:10.1016/j.antiviral.2005.07.002
Livingston, Loach, Wilson, Tannock, Baird, Gut commensal Lactobacillus reuteri 100-23 stimulates an immunoregulatory response, Immunol. Cell Biol, doi:10.1038/icb.2009.71
Louca, Murray, Klaser, Graham, Mazidi et al., Modest effects of dietary supplements during the COVID-19 pandemic: Insights from 445 850 users of the COVID-19 Symptom Study app, BMJ Nutr. Prev. Health, doi:10.1136/bmjnph-2021-000250
Lu, Yan, Liu, Ding, Chen et al., Probiotics in preventing and treating chemotherapy-induced diarrhoea: A meta-analysis, Asia Pac. J. Clin. Nutr
Lv, Qiu, Chen, Zheng, Jin et al., Apigenin Inhibits Enterovirus 71 Replication Through Suppressing Viral IRES Activity and Modulating Cellular JNK Pathway, Antivir. Res, doi:10.1016/j.antiviral.2014.06.004
Ma, Gu, Hou, Zhang, Bai et al., Incidence, clinical characteristics and prognostic factor of patients with COVID-19: A systematic review and meta-analysis, MedRxIV, doi:10.1101/2020.03.17.20037572
Macfarlane, Cleary, Bahrami, Reynolds, Macfarlane, Synbiotic consumption changes the metabolism and composition of the gut microbiota in older people and modifies inflammatory processes: A randomised, double-blind, placebocontrolled crossover study, Aliment. Pharmacol. Ther, doi:10.1111/apt.12453
Mammen, Sethi, COPD and the microbiome, Respiratory, doi:10.1111/resp.12732
Martinez, Kahana, Ghuman, Wilson, Wilson et al., Unhealthy Lifestyle and Gut Dysbiosis: A Better Understanding of the Effects of Poor Diet and Nicotine on the Intestinal Microbiome, Front. Endocrinol, doi:10.3389/fendo.2021.667066
Martinez, Moreno, Effect of Resveratrol, a Natural Polyphenolic Compound, on Reactive Oxygen Species and Prostaglandin Production, Biochem. Pharmacol, doi:10.1016/S0006-2952(99)00380-9
Meijnikman, Gerdes, Nieuwdorp, Herrema, Evaluating causality of gut microbiota in obesity and diabetes in humans, Endocr. Rev, doi:10.1210/er.2017-00192
Meijvis, Hardeman, Remmelts, Heijligenberg, Van Velzen-Blad et al., Dexamethasone and length of hospital stay in patients with community-acquired pneumonia: A randomised, double-blind, placebo-controlled trial, Lancet, doi:10.1016/S0140-6736(11)60607-7
Morrison, Preston, Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism, Gut Microbes, doi:10.1080/19490976.2015.1134082
Morshedi, Hashemi, Moazzen, Sahebkar, Hosseinifard, Immunomodulatory and anti-inflammatory effects of probiotics in multiple sclerosis: A systematic review, J. Neuroinflamm, doi:10.1186/s12974-019-1611-4
Namasivayam, Sher, Glickman, Wipperman, The Microbiome and Tuberculosis: Early Evidence for Cross Talk, Am. Soc. Microbiol, doi:10.1128/mBio.01420-18
Nencioni, Iuvara, Aquilano, Ciriolo, Cozzolino et al., Influenza A Virus Replication is Dependent on an Antioxidant Pathway That Involves GSH and Bcl-2, J. Am. Soc. Exp. Biol
Ng, Tilg, COVID-19 and the gastrointestinal tract: More than meets the eye, Gut, doi:10.1136/gutjnl-2020-321195
Nishida, Inoue, Inatomi, Bamba, Naito et al., Gut microbiota in the pathogenesis of inflammatory bowel disease, Clin. J. Gastroenterol, doi:10.1007/s12328-017-0813-5
Nobaek, Johansson, Molin, Ahrne, Jeppsson, Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome, Am. J. Gastroenterol, doi:10.1111/j.1572-0241.2000.02015.x
Olaimat, Aolymat, Al-Holy, Ayyash, Abu Ghoush et al., The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19, Nature, doi:10.1038/s41538-020-00078-9
Palamara, Nencioni, Aquilano, De Chiara, Hernandez et al., Inhibition of Influenza A Virus Replication by Resveratrol, J. Infect. Dis, doi:10.1086/429694
Park, Kwon, Yoo, Choi, Ahn, Antiviral activity and possible mode of action of ellagic acid identified in Lagerstroemia speciosa leaves toward human rhinoviruses, BMC Complementary Med. Ther, doi:10.1186/1472-6882-14-171
Plaza-Diaz, Ruiz-Ojeda, Gil-Campos, Gil, Mechanisms of action of probiotics, Adv. Nutr, doi:10.1093/advances/nmy063
Poljsak, Strategies for reducing or preventing the generation of oxidative stress, Oxidative Med. Cell. Longev, doi:10.1155/2011/194586
Pontin, Schwannauer, Tai, Kinderman, A UK validation of a general measure of subjective well-being: The modified BBC subjective well-being scale (BBC-SWB), Health Qual. Life Outcomes, doi:10.1186/1477-7525-11-150
Pourhossein, Moravejolahkami, Probiotics in viral infections, with a focus on COVID-19: A systematic review, Authorea, doi:10.22541/au.158999387.76467979
Powanda, Rainsford, A toxicological investigation of a celery seed extract having anti-inflammatory activity, Inflammopharmacology, doi:10.1007/s10787-010-0049-1
Powanda, Whitehouse, Rainsford, Celery Seed and Related Extracts with Antiarthritic, Antiulcer, and Antimicrobial Activities, Prog. Drug Res
Preidis, Weizman, Kashyap, Morgan, AGA Technical Review on the Role of Probiotics in the Management of Gastrointestinal Disorders, Gastroenterology, doi:10.1053/j.gastro.2020.05.060
Qian, Fan, Qian, Zhang, Wei et al., Apigenin Restricts FMDV Infection and Inhibits Viral IRES Driven Translational Activity, Viruses, doi:10.3390/v7041613
Rattis, Ramos, Celes, Curcumin as a Potential Treatment for COVID-19, Front. Pharmacol
Reinold, Farahpour, Fehring, Dolff, Konik et al., A Pro-Inflammatory Gut Microbiome Characterizes SARS-CoV-2 Infected Patients and a Reduction in the Connectivity of an Anti-Inflammatory Bacterial Network Associates with Severe COVID-19, Front. Cell Infect. Microbiol, doi:10.3389/fcimb.2021.747816
Rerksuppaphol, Rerksuppaphol, Randomized controlled trial of probiotics to reduce common cold in schoolchildren, Paediatr. Int, doi:10.1111/j.1442-200X.2012.03647.x
Ristow, Zarse, How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis), Exp. Gerontol, doi:10.1016/j.exger.2010.03.014
Rossi, Sacco, Capizzi, Mastromarino, Can Resveratrol-Inhaled Formulations Be Considered Potential Adjunct Treatments for COVID-19? Front, Immunol, doi:10.3389/fimmu.2021.670955
Salaris, Scarpa, Elli, Bertolini, Guglielmetti et al., Lacticaseibacillus enhances the lactoferrin anti-SARS-CoV-2 response in Caco-2 cells, Gut Microbes, doi:10.1080/19490976.2021.1961970
Salminen, Tynkkynen, Rautelin, Saxelin, Vaara et al., Lactobacillus bacteremia during a rapid increase in probiotic use of Lactobacillus rhamnosus GG in Finland, Clin. Infect. Dis, doi:10.1086/342912
Sanders, Akkermans, Haller, Hammerman, Heimbach et al., Safety assessment of probiotics for human use, Gut Microbes, doi:10.4161/gmic.1.3.12127
Shang, Sun, Vitamin, Vdr, Probiotics, and Gastrointestinal Diseases, Curr. Med. Chem, doi:10.2174/0929867323666161202150008
Shen, Smith, Lo, Chyu, Dunn et al., Dietary polyphenols and mechanisms of osteoarthritis, J. Nutr. Biochem, doi:10.1016/j.jnutbio.2012.04.001
Singh, Chopra, Kuhad, Kaur, Role of Lactobacillus acidophilus loaded floating beads in chronic fatigue syndrome: Behavioural and biochemical evidences, Neurogastroenterol. Motil, doi:10.1111/j.1365-2982.2011.01861.x
Singh, Rao, Probiotics: A potential immunomodulator in COVID-19 infection management, Nutr. Res, doi:10.1016/j.nutres.2020.12.014
Singh, Rawat, Alwakeel, Sharif, Al Khodor, The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals, Sci. Rep, doi:10.1038/s41598-020-77806-4
Su, Jia, Li, Zhou, Probiotics for the Prevention of Ventilator-Associated Pneumonia: A Meta-Analysis of Randomized Controlled Trials, Respir. Care, doi:10.4187/respcare.07097
Sullivan, Nord, Evengård, Effect of supplement with lactic-acid producing bacteria on fatigue and physical activity in patients with chronic fatigue syndrome, Nutr. J, doi:10.1186/1475-2891-8-4
Tang, Cosentino, Lee, Apigenin-7-O-β-D-glucopyranoside, an anti-HIV principle from Kummerowia striata, Bioorganic Med. Chem. Lett, doi:10.1016/0960-894X(94)80015-4
Teixeira, Valente, Casal, Marques, Moreira, Antioxidant vitamins do not prevent post exercise peroxidation and may delay muscle recovery, Med. Sci. Sports Exerc, doi:10.1249/MSS.0b013e31819fe8e3
Thomas, Aldous, Forsyth, Chater, Williams, The Influence of a blend of Probiotic Lactobacillus and Prebiotic Inulin on the Duration and Severity of Symptoms among Individuals with COVID-19, Infect. Dis. Diagn. Treat
Thomas, Versalovic, Probiotics-host communication: Modulation of signaling pathways in the intestine, Gut Microbes, doi:10.4161/gmic.1.3.11712
Thomas, Yang, Zollman, Williams, Phytochemicals in Cancer Management, Curr. Res. Complement. Altern. Med
Tito, Colantuono, Pirone, Pedone, Intartaglia et al., Pomegranate Peel Extract as an Inhibitor of SARS-CoV-2 Spike Binding to Human ACE2 Receptor (in vitro): A Promising Source of Novel Antiviral Drugs, Front. Chem, doi:10.3389/fchem.2021.638187
Uchide, Toyoda, Antioxidant therapy as a potential approach to severe influenza-associated complications, Molecules, doi:10.3390/molecules16032032
Van Baarlen, Troost, Van Der Meer, Hooiveld, Boekschoten et al., Human mucosal in vivo transcriptome responses to three Lactobacilli indicate how probiotics may modulate human cellular pathways, Proc. Natl. Acad. Sci, doi:10.1073/pnas.1000079107
Van Heul, Planer, Kau, The human microbiota and asthma, Clin. Rev. Allergy Immunol, doi:10.1007/s12016-018-8719-7
Waki, Mastumoto, Fuku, Suganuma, Effects of probiotic Lactobacillus brevis KB290 on incidence of influenza infection among schoolchildren: An open-label pilot study, Lett. Appl. Microbiol, doi:10.1111/lam.12340
Wan, Lie, Shen, Zou, Hou et al., Enteric involvement in hospitalised patients with COVID-19 outside Wuhan, Lancet Gastroenterol. Hepatol, doi:10.1016/S2468-1253(20)30118-7
Wang, Wu, Wang, Xu, Mei et al., Antioxidant properties of probiotic bacteria, Nutrients, doi:10.3390/nu9050521
Wang, Zhu, Qin, Gut microbiota modulation on intestinal mucosal adaptive immunity, J. Immunol. Res
Williamson, Burns, Gossard, Pizano, ; E Dolan et al., Probiotics and Disease: A Comprehensive Summary-Part 3, Cardiometabolic Disease and Fatigue Syndromes, Integr. Med. A Clin. J
Wischmeyer, Tang, Ren, Bohannon, Ramirez et al., Daily Lactobacillus Probiotic versus Placebo in COVID-19-Exposed Household Contacts (PROTECT-EHC): A Randomized Clinical Trial, doi:10.1101/2022.01.04.21268275
Wu, Yoon, Zhang, Lu, Xia et al., Vitamin D receptor pathway is required for probiotic protection in colitis, Am. J. Physiol. Gastrointest. Liver Physiol, doi:10.1152/ajpgi.00105.2015
Xu, Cai, Shen, Ni, Chen et al., Management of coronavirus disease-19 (COVID-19): The Zhejiang experience, J. Zhejiang Univ. Med. Sci
Yeoh, Zuo, Lui, Zhang, Liu et al., Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19, Gut, doi:10.1136/gutjnl-2020-323020
Yoon, Wu, Zhang, Lu, Petrof et al., Probiotic regulation of vitamin D receptor in intestinal inflammation, Gastroenterology, doi:10.1016/S0016-5085(11)60075-9
Yurkovetskiy, Burrows, Khan, Graham, Volchkov et al., Gender bias in autoimmunity is influenced by microbiota, Immunity, doi:10.1016/j.immuni.2013.08.013
Zakaryan, Arabyan, Oo, Zandi, Flavonoids: Promising natural compounds against viral infections, Arch. Virol, doi:10.1007/s00705-017-3417-y
DOI record:
{
"DOI": "10.3390/covid2040031",
"ISSN": [
"2673-8112"
],
"URL": "http://dx.doi.org/10.3390/covid2040031",
"abstract": "<jats:p>Gut microflora dysbiosis affects the majority of individuals after COVID-19, contributing to both gastro-intestinal (GI) and non-GI symptoms. Natural phytochemicals have reported anti-viral properties and favourable effects on inflammatory and oxidative pathways, both important for tissue damage post-viral pneumonia. This study involved 147 participants with symptomatic COVID-19, randomised to receive a placebo (P) or a phytochemical-rich concentrated food capsule (PC) in addition to a pre/probiotic lactobacillus capsule. Participants taking the PC had an almost two-fold reduction in mean fatigue scores compared to P [p = 0.02], a three-fold reduction in cough score and more than a double improvement in overall well-being scores [p = 0.02]. Two (1.5%) participants reported mild, increased bloating which they felt was attributable to the capsules, although GI symptoms improved in 25 of 31 participants (82%) who reported them at baseline. Sedentary, older, previously hospitalised men with GI symptoms had a statistically significantly improvement among those given the probiotic. Although some participants with early disease would have improved spontaneously, such a rapid improvement observed in the majority of participants, who had been suffering for an average of 108 days, was clinically relevant and welcomed, especially among those more likely to have pre-existing gut dysbiosis. We are now evaluating whether this blend could also enhance antibody titres post-COVID-19 vaccination.</jats:p>",
"alternative-id": [
"covid2040031"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-3824-5615",
"affiliation": [],
"authenticated-orcid": false,
"family": "Thomas",
"given": "Robert",
"sequence": "first"
},
{
"affiliation": [],
"family": "Williams",
"given": "Madeleine",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9159-4646",
"affiliation": [],
"authenticated-orcid": false,
"family": "Aldous",
"given": "Jeffrey",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3710-9328",
"affiliation": [],
"authenticated-orcid": false,
"family": "Yanagisawa",
"given": "Yuuki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kumar",
"given": "Rajeev",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Forsyth",
"given": "Rachel",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9043-2565",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chater",
"given": "Angel",
"sequence": "additional"
}
],
"container-title": "COVID",
"container-title-short": "COVID",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
3,
22
]
],
"date-time": "2022-03-22T18:55:35Z",
"timestamp": 1647975335000
},
"deposited": {
"date-parts": [
[
2022,
3,
22
]
],
"date-time": "2022-03-22T19:25:08Z",
"timestamp": 1647977108000
},
"indexed": {
"date-parts": [
[
2022,
4,
6
]
],
"date-time": "2022-04-06T02:17:06Z",
"timestamp": 1649211426528
},
"is-referenced-by-count": 0,
"issue": "4",
"issued": {
"date-parts": [
[
2022,
3,
22
]
]
},
"journal-issue": {
"issue": "4",
"published-online": {
"date-parts": [
[
2022,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
22
]
],
"date-time": "2022-03-22T00:00:00Z",
"timestamp": 1647907200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2673-8112/2/4/31/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "433-449",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
3,
22
]
]
},
"published-online": {
"date-parts": [
[
2022,
3,
22
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.3390/nu7010017",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.1101/2020.03.17.20037572",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.4161/gmic.1.3.11712",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1016/j.arcmed.2021.03.002",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.1038/nrmicro.2016.142",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.1136/gutjnl-2020-323020",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"article-title": "Management of coronavirus disease-19 (COVID-19): The Zhejiang experience",
"author": "Xu",
"first-page": "147",
"journal-title": "J. Zhejiang Univ. Med. Sci.",
"key": "ref7",
"volume": "49",
"year": "2020"
},
{
"DOI": "10.1136/gutjnl-2021-324090",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1016/S0140-6736(20)32656-8",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1093/cid/ciaa709",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1136/gutjnl-2020-321195",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.1016/j.jnutbio.2012.04.001",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1016/j.virusres.2020.198018",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1155/2019/4735040",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1016/j.antiviral.2005.07.002",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1038/s41538-020-00078-9",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1016/S2213-2600(15)00427-0",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.3390/molecules16032032",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.3389/fcimb.2020.00009",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1128/mBio.01420-18",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"article-title": "Lung microbiota in the acute respiratory disease: From coronavirus to metabolomics",
"author": "Fanos",
"first-page": "90",
"journal-title": "J. Paediatr. Neonatal Individ. Med.",
"key": "ref21",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1016/j.mehy.2020.110102",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.1111/cmi.12966",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1016/S2468-1253(20)30118-7",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.3389/fcimb.2021.747816",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.3389/fmicb.2018.02147",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1007/s12016-018-8719-7",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.1111/resp.12732",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.3389/fendo.2021.667066",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.1093/cdn/nzy005",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"article-title": "Phytochemicals in Cancer Management",
"author": "Thomas",
"first-page": "1",
"journal-title": "Curr. Res. Complement. Altern. Med.",
"key": "ref31",
"volume": "105",
"year": "2017"
},
{
"article-title": "The Influence of a blend of Probiotic Lactobacillus and Prebiotic Inulin on the Duration and Severity of Symptoms among Individuals with COVID-19",
"author": "Thomas",
"first-page": "1",
"journal-title": "Infect. Dis. Diagn. Treat.",
"key": "ref32",
"volume": "5",
"year": "2021"
},
{
"DOI": "10.1111/j.1572-0241.2000.02015.x",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.1155/2018/8063647",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1186/s12974-019-1611-4",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"article-title": "Probiotics for prevention of necrotizing enterocolitis in preterm infants",
"author": "AlFaleh",
"first-page": "584",
"journal-title": "Cochrane Database Syst. Rev.",
"key": "ref36",
"volume": "4",
"year": "2014"
},
{
"DOI": "10.1111/apt.12453",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.1136/gut.51.5.659",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.1159/000342079",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.1023/A:1010979225018",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.1093/advances/nmy063",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1038/icb.2009.71",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.3109/09637486.2013.836738",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"DOI": "10.3390/ijerph15081679",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"DOI": "10.1007/s12328-017-0813-5",
"doi-asserted-by": "publisher",
"key": "ref45"
},
{
"DOI": "10.1016/j.ebiom.2019.11.051",
"doi-asserted-by": "publisher",
"key": "ref46"
},
{
"DOI": "10.1210/er.2017-00192",
"doi-asserted-by": "publisher",
"key": "ref47"
},
{
"DOI": "10.3233/JAD-161141",
"doi-asserted-by": "publisher",
"key": "ref48"
},
{
"DOI": "10.1073/pnas.1000079107",
"doi-asserted-by": "publisher",
"key": "ref49"
},
{
"DOI": "10.3390/nu10080988",
"doi-asserted-by": "publisher",
"key": "ref50"
},
{
"DOI": "10.1016/j.nutres.2020.12.014",
"doi-asserted-by": "publisher",
"key": "ref51"
},
{
"DOI": "10.3390/nu9050521",
"doi-asserted-by": "publisher",
"key": "ref52"
},
{
"DOI": "10.1210/jc.2012-4262",
"doi-asserted-by": "publisher",
"key": "ref53"
},
{
"DOI": "10.1038/s41598-020-77806-4",
"doi-asserted-by": "publisher",
"key": "ref54"
},
{
"DOI": "10.2174/0929867323666161202150008",
"doi-asserted-by": "publisher",
"key": "ref55"
},
{
"DOI": "10.1016/S0016-5085(11)60075-9",
"doi-asserted-by": "publisher",
"key": "ref56"
},
{
"DOI": "10.1152/ajpgi.00105.2015",
"doi-asserted-by": "publisher",
"key": "ref57"
},
{
"DOI": "10.1016/j.clnu.2018.10.028",
"doi-asserted-by": "publisher",
"key": "ref58"
},
{
"article-title": "The possible role of vitamin D in suppressing cytokine storm and associated mortality in COVID-19 patients",
"author": "Daneshkhah",
"first-page": "1",
"journal-title": "MedRxiv",
"key": "ref59",
"volume": "78",
"year": "2020"
},
{
"DOI": "10.1002/jmv.26832",
"doi-asserted-by": "publisher",
"key": "ref60"
},
{
"DOI": "10.1007/s12602-014-9162-6",
"doi-asserted-by": "publisher",
"key": "ref61"
},
{
"DOI": "10.1080/19490976.2021.1961970",
"doi-asserted-by": "publisher",
"key": "ref62"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106118",
"doi-asserted-by": "publisher",
"key": "ref63"
},
{
"DOI": "10.4172/2161-0517.1000e107",
"doi-asserted-by": "publisher",
"key": "ref64"
},
{
"DOI": "10.1046/j.1365-2672.2003.02026.x",
"doi-asserted-by": "publisher",
"key": "ref65"
},
{
"DOI": "10.1016/j.ajic.2013.04.005",
"doi-asserted-by": "publisher",
"key": "ref66"
},
{
"DOI": "10.1111/j.1442-200X.2012.03647.x",
"doi-asserted-by": "publisher",
"key": "ref67"
},
{
"DOI": "10.1111/lam.12340",
"doi-asserted-by": "publisher",
"key": "ref68"
},
{
"DOI": "10.4082/kjfm.2013.34.1.2",
"doi-asserted-by": "publisher",
"key": "ref69"
},
{
"DOI": "10.1038/s41598-019-39602-7",
"doi-asserted-by": "publisher",
"key": "ref70"
},
{
"DOI": "10.1016/j.ejphar.2014.05.028",
"doi-asserted-by": "publisher",
"key": "ref71"
},
{
"DOI": "10.4187/respcare.07097",
"doi-asserted-by": "publisher",
"key": "ref72"
},
{
"DOI": "10.1007/978-3-0348-0927-6_4",
"article-title": "Celery Seed and Related Extracts with Antiarthritic, Antiulcer, and Antimicrobial Activities",
"author": "Powanda",
"doi-asserted-by": "crossref",
"first-page": "133",
"journal-title": "Prog. Drug Res.",
"key": "ref73",
"volume": "70",
"year": "2015"
},
{
"DOI": "10.1007/s10787-010-0049-1",
"doi-asserted-by": "publisher",
"key": "ref74"
},
{
"DOI": "10.3389/fnmol.2015.00024",
"doi-asserted-by": "publisher",
"key": "ref75"
},
{
"DOI": "10.1021/np050327j",
"doi-asserted-by": "publisher",
"key": "ref76"
},
{
"DOI": "10.1142/S0192415X0900703X",
"doi-asserted-by": "publisher",
"key": "ref77"
},
{
"DOI": "10.3389/fimmu.2021.670955",
"doi-asserted-by": "publisher",
"key": "ref78"
},
{
"DOI": "10.1016/S0006-2952(99)00380-9",
"doi-asserted-by": "publisher",
"key": "ref79"
},
{
"DOI": "10.1096/fj.02-0508fje",
"doi-asserted-by": "publisher",
"key": "ref80"
},
{
"DOI": "10.1016/j.pharmthera.2020.107613",
"doi-asserted-by": "publisher",
"key": "ref81"
},
{
"DOI": "10.1042/BST0380050",
"doi-asserted-by": "publisher",
"key": "ref82"
},
{
"DOI": "10.1016/0960-894X(94)80015-4",
"doi-asserted-by": "publisher",
"key": "ref83"
},
{
"DOI": "10.1089/aid.1996.12.39",
"doi-asserted-by": "publisher",
"key": "ref84"
},
{
"DOI": "10.3390/antiox9100936",
"doi-asserted-by": "publisher",
"key": "ref85"
},
{
"DOI": "10.1155/2011/194586",
"doi-asserted-by": "publisher",
"key": "ref86"
},
{
"DOI": "10.1016/j.exger.2010.03.014",
"doi-asserted-by": "publisher",
"key": "ref87"
},
{
"DOI": "10.1249/MSS.0b013e31819fe8e3",
"doi-asserted-by": "publisher",
"key": "ref88"
},
{
"article-title": "Effects of vitamin E supplementation on recovery from repeated bouts of resistance exercise",
"author": "Avery",
"first-page": "801",
"journal-title": "J. Strength Cond. Res.",
"key": "ref89",
"volume": "17",
"year": "2003"
},
{
"DOI": "10.1006/niox.1996.0113",
"doi-asserted-by": "publisher",
"key": "ref90"
},
{
"DOI": "10.1016/j.celrep.2019.06.018",
"doi-asserted-by": "publisher",
"key": "ref91"
},
{
"DOI": "10.1161/HYPERTENSIONAHA.113.02044",
"doi-asserted-by": "publisher",
"key": "ref92"
},
{
"DOI": "10.1016/j.antiviral.2005.02.007",
"doi-asserted-by": "publisher",
"key": "ref93"
},
{
"DOI": "10.1186/1472-6882-14-171",
"doi-asserted-by": "publisher",
"key": "ref94"
},
{
"DOI": "10.3389/fchem.2021.638187",
"doi-asserted-by": "publisher",
"key": "ref95"
},
{
"DOI": "10.3389/fimmu.2020.01451",
"doi-asserted-by": "publisher",
"key": "ref96"
},
{
"DOI": "10.1086/429694",
"doi-asserted-by": "publisher",
"key": "ref97"
},
{
"DOI": "10.3390/v12111242",
"doi-asserted-by": "publisher",
"key": "ref98"
},
{
"DOI": "10.3389/fphar.2021.675287",
"doi-asserted-by": "publisher",
"key": "ref99"
},
{
"DOI": "10.1016/j.biopha.2016.03.037",
"doi-asserted-by": "publisher",
"key": "ref100"
},
{
"DOI": "10.1055/s-2003-43207",
"article-title": "A New Anti-HIV Flavonoid Glucuronide from Chrysanthemum Morifolium",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "859",
"journal-title": "Planta Medica",
"key": "ref101",
"volume": "69",
"year": "2003"
},
{
"DOI": "10.1016/j.antiviral.2014.06.004",
"doi-asserted-by": "publisher",
"key": "ref102"
},
{
"DOI": "10.3390/v7041613",
"doi-asserted-by": "publisher",
"key": "ref103"
},
{
"DOI": "10.1007/s00705-017-3417-y",
"doi-asserted-by": "publisher",
"key": "ref104"
},
{
"DOI": "10.1007/s11101-021-09759-z",
"doi-asserted-by": "publisher",
"key": "ref105"
},
{
"DOI": "10.3390/molecules26226825",
"doi-asserted-by": "publisher",
"key": "ref106"
},
{
"DOI": "10.1016/j.jff.2020.104169",
"doi-asserted-by": "publisher",
"key": "ref107"
},
{
"DOI": "10.3390/ijms21155423",
"doi-asserted-by": "publisher",
"key": "ref108"
},
{
"DOI": "10.1016/j.freeradbiomed.2019.02.023",
"doi-asserted-by": "publisher",
"key": "ref109"
},
{
"DOI": "10.1080/19490976.2015.1134082",
"doi-asserted-by": "publisher",
"key": "ref110"
},
{
"DOI": "10.1016/j.cell.2016.05.041",
"doi-asserted-by": "publisher",
"key": "ref111"
},
{
"DOI": "10.1021/jf101475m",
"doi-asserted-by": "publisher",
"key": "ref112"
},
{
"DOI": "10.1136/gutjnl-2020-322670",
"doi-asserted-by": "publisher",
"key": "ref113"
},
{
"DOI": "10.1038/nature12820",
"doi-asserted-by": "publisher",
"key": "ref114"
},
{
"DOI": "10.1186/1477-7525-11-150",
"doi-asserted-by": "publisher",
"key": "ref115"
},
{
"DOI": "10.1016/0022-3999(93)90081-P",
"doi-asserted-by": "publisher",
"key": "ref116"
},
{
"DOI": "10.1136/gutjnl-2020-321013",
"doi-asserted-by": "publisher",
"key": "ref117"
},
{
"DOI": "10.3389/fpubh.2020.00186",
"doi-asserted-by": "publisher",
"key": "ref118"
},
{
"DOI": "10.22541/au.158999387.76467979",
"doi-asserted-by": "publisher",
"key": "ref119"
},
{
"DOI": "10.5195/ijms.2020.486",
"doi-asserted-by": "publisher",
"key": "ref120"
},
{
"DOI": "10.1080/19490976.2021.2018899",
"doi-asserted-by": "publisher",
"key": "ref121"
},
{
"DOI": "10.1101/2022.01.04.21268275",
"doi-asserted-by": "publisher",
"key": "ref122"
},
{
"DOI": "10.1136/bmjnph-2021-000250",
"doi-asserted-by": "publisher",
"key": "ref123"
},
{
"DOI": "10.1016/S0140-6736(11)60607-7",
"doi-asserted-by": "publisher",
"key": "ref124"
},
{
"DOI": "10.3889/oamjms.2015.005",
"doi-asserted-by": "publisher",
"key": "ref125"
},
{
"DOI": "10.1007/s42399-020-00341-w",
"doi-asserted-by": "publisher",
"key": "ref126"
},
{
"DOI": "10.1016/j.immuni.2013.08.013",
"doi-asserted-by": "publisher",
"key": "ref127"
},
{
"DOI": "10.1016/j.tins.2013.10.003",
"doi-asserted-by": "publisher",
"key": "ref128"
},
{
"DOI": "10.1186/s40168-016-0171-4",
"doi-asserted-by": "publisher",
"key": "ref129"
},
{
"article-title": "Probiotics and Disease: A Comprehensive Summary-Part 3, Cardiometabolic Disease and Fatigue Syndromes",
"author": "Williamson",
"first-page": "30",
"journal-title": "Integr. Med. A Clin. J.",
"key": "ref130",
"volume": "16",
"year": "2017"
},
{
"DOI": "10.1089/jmf.2014.7000",
"doi-asserted-by": "publisher",
"key": "ref131"
},
{
"DOI": "10.1186/1475-2891-8-4",
"doi-asserted-by": "publisher",
"key": "ref132"
},
{
"DOI": "10.1111/j.1365-2982.2011.01861.x",
"doi-asserted-by": "publisher",
"key": "ref133"
},
{
"DOI": "10.1093/cid/civ085",
"doi-asserted-by": "publisher",
"key": "ref134"
},
{
"DOI": "10.1016/j.ijfoodmicro.2007.08.015",
"doi-asserted-by": "publisher",
"key": "ref135"
},
{
"DOI": "10.1053/j.gastro.2020.05.060",
"doi-asserted-by": "publisher",
"key": "ref136"
},
{
"article-title": "Safety of probiotics used to reduce risk and prevent or treat disease",
"author": "Hempel",
"first-page": "1",
"journal-title": "Evid. Rep. Technol. Assess.",
"key": "ref137",
"volume": "200",
"year": "2011"
},
{
"DOI": "10.4161/gmic.1.3.12127",
"doi-asserted-by": "publisher",
"key": "ref138"
},
{
"DOI": "10.1086/368080",
"doi-asserted-by": "publisher",
"key": "ref139"
},
{
"DOI": "10.1111/j.1574-6976.2006.00020.x",
"doi-asserted-by": "publisher",
"key": "ref140"
},
{
"article-title": "Probiotics in preventing and treating chemotherapy-induced diarrhoea: A meta-analysis",
"author": "Lu",
"first-page": "701",
"journal-title": "Asia Pac. J. Clin. Nutr.",
"key": "ref141",
"volume": "28",
"year": "2019"
},
{
"DOI": "10.1086/342912",
"doi-asserted-by": "publisher",
"key": "ref142"
}
],
"reference-count": 142,
"references-count": 142,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2673-8112/2/4/31"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "A Randomised, Double-Blind, Placebo-Controlled Trial Evaluating Concentrated Phytochemical-Rich Nutritional Capsule in Addition to a Probiotic Capsule on Clinical Outcomes among Individuals with COVID-19—The UK Phyto-V Study",
"type": "journal-article",
"volume": "2"
}
