Daily Lactobacillus Probiotic versus Placebo in COVID-19-Exposed Household Contacts (PROTECT-EHC): A Randomized Clinical Trial
et al., medRxiv, doi:10.1101/2022.01.04.21268275, PROTECT-EHC, NCT04399252, Jan 2022
Probiotics for COVID-19
20th treatment shown to reduce risk in
March 2021, now with p = 0.00000044 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
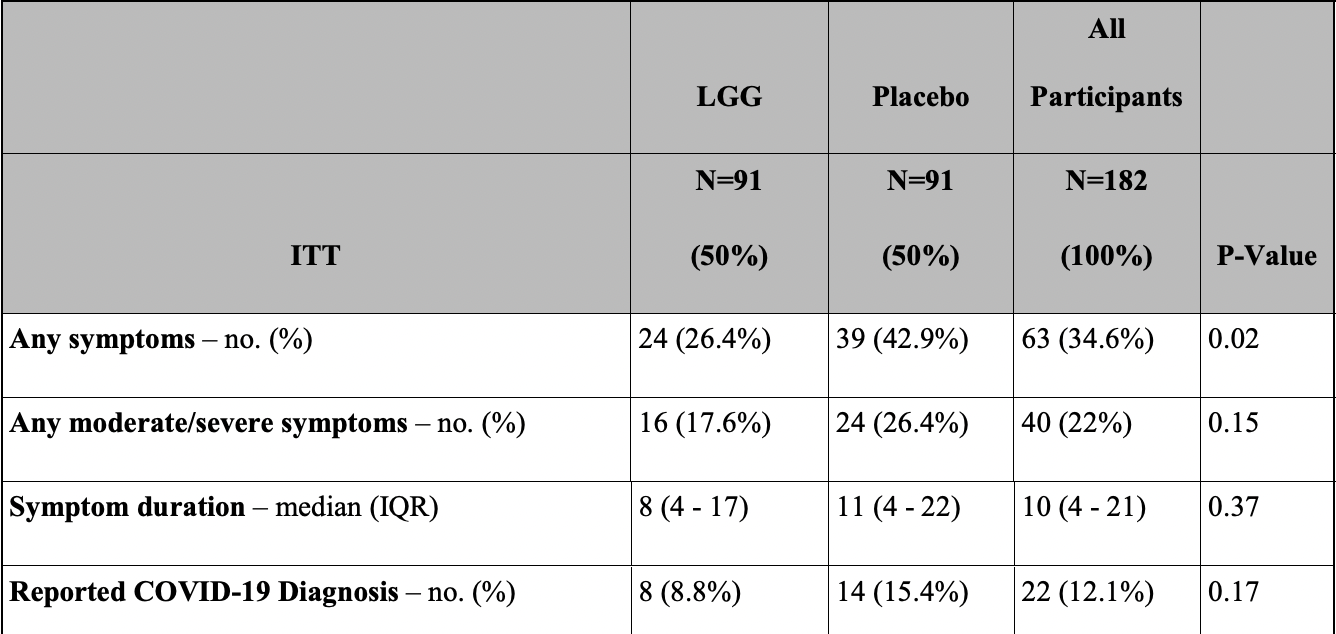
RCT 182 COVID-19 exposed patients, 91 treated with daily probiotic Lactobacillus rhamnosus GG starting a median of 3 days from exposure, showing lower symptomatic COVID-19 with treatment. There were no hospitalizations or deaths.
Probiotic efficacy depends on the specific strains used. Specific microbes may decrease or increase COVID-19 risk1.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments2.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of moderate/severe case, 33.3% lower, RR 0.67, p = 0.15, treatment 16 of 91 (17.6%), control 24 of 91 (26.4%), NNT 11.
|
|
risk of symptomatic case, 38.5% lower, RR 0.62, p = 0.02, treatment 24 of 91 (26.4%), control 39 of 91 (42.9%), NNT 6.1, primary outcome.
|
|
recovery time, 27.3% lower, relative time 0.73, p = 0.37, treatment 91, control 91.
|
|
risk of case, 42.9% lower, RR 0.57, p = 0.17, treatment 8 of 91 (8.8%), control 14 of 91 (15.4%), NNT 15.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Wischmeyer et al., 5 Jan 2022, Double Blind Randomized Controlled Trial, USA, preprint, 21 authors, study period 24 June, 2020 - 8 July, 2021, trial NCT04399252 (history) (PROTECT-EHC).
Daily Lactobacillus Probiotic versus Placebo in COVID-19-Exposed Household Contacts (PROTECT-EHC): A Randomized Clinical Trial
doi:10.1101/2022.01.04.21268275
Importance: The COVID-19 pandemic continues to pose unprecedented challenges to worldwide health. While vaccines are effective, supplemental strategies to mitigate the spread and severity of COVID-19 are urgently needed. Emerging evidence suggests susceptibility to infections, including respiratory tract infections, may be reduced by probiotic interventions; therefore, probiotics may be a low-risk, widely implementable modality to mitigate risk of COVID-19 disease, particularly in areas with low vaccine availability and/or uptake. Objective: To determine whether daily probiotic Lactobacillus rhamnosus GG (LGG) is effective in preventing development of symptoms of illness within 28 days of COVID-19 exposure. Design: This randomized, double-blind, placebo-controlled trial across the United States (PROTECT-EHC) enrolled in 2020-2021. Participants were followed for 60 days. Setting: Describe the study setting to assist readers to determine the applicability of the report to other circumstances, for example, multicenter, population-based, primary care or referral center(s), etc. Participants: Participants included individuals > 1 year of age with a household contact with a recent (≤ 7 days) diagnosis of COVID-19. 182 participants were enrolled and randomized during the study period. Intervention: Participants were randomized to receive daily oral LGG or microcrystalline cellulose placebo for 28 days.
Main Outcomes and Measures: The primary outcome was development of symptoms within 28 days of exposure to a COVID-19-infected household contact. Stool was collected to evaluate for changes in microbiome structure. Results: 182 participants were enrolled and randomized during the study period. Intention-to-treat analysis showed that participants randomized to LGG were less likely to develop symptoms versus those randomized to placebo (26.4% vs. 42.9%, p=0.02). Further, LGG was associated with a statistically significant reduction in COVID-19 diagnosis (log rank p=0.049) via time-to-event analysis. Overall incidence of COVID-19 diagnosis did not significantly differ between LGG and placebo groups (8.8% vs. 15.4%, p=0.17). LGG was well-tolerated with no increased side effects versus placebo. Placebo recipients were more likely to stop the study product, temporarily or permanently, due to symptoms attributed to the study product (5.5% vs. 0%, p = 0.02).
Conclusions and Relevance: Our study suggests that LGG is well-tolerated and is associated with prolonged time to development of COVID-19 infection, reduced incidence of symptoms, and changes to gut microbiome structure when used as post-exposure prophylaxis within 7 days after exposure. This preliminary work may inform the approach to prevention of COVID-19, particularly in underdeveloped nations where Lactobacillus probiotics have already been
References
Burke, Midgley, Dratch, Active Monitoring of Persons Exposed to Patients with Confirmed COVID-19 -United States, January, MMWR Morb Mortal Wkly Rep
Callahan, Mcmurdie, Rosen, Han, Johnson et al., DADA2: High-resolution sample inference from Illumina amplicon data, Nat Methods
Davison, Wischmeyer, Probiotic and synbiotic therapy in the critically ill: State of the art, Nutrition
Dolgin, COVID vaccine immunity is waning -how much does that matter?, Nature
Farshbaf-Khalili, Farajnia, Pourzeinali, Shakouri, Salehi-Pourmehr, The effect of nanomicelle curcumin supplementation and Nigella sativa oil on the expression level of miRNA-21, miRNA-422a, and miRNA-503 gene in postmenopausal women with low bone mass density: A randomized, triple-blind, placebocontrolled clinical trial with factorial design, Phytother Res
Hao, Dong, Wu, Probiotics for preventing acute upper respiratory tract infections, Cochrane Database of Systematic Reviews
Hao, Dong, Wu, Probiotics for preventing acute upper respiratory tract infections. The Cochrane database of systematic reviews
Harris, Taylor, Thielke, Payne, Gonzalez et al., Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform
Hibberd, Yde, Ziegler, Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults, Benef Microbes
Khailova, Baird, Rush, Barnes, Wischmeyer, Lactobacillus rhamnosus GG treatment improves intestinal permeability and modulates inflammatory response and homeostasis of spleen and colon in experimental model of Pseudomonas aeruginosa pneumonia, Clin Nutr
Khailova, Baird, Rush, Mcnamee, Wischmeyer, Lactobacillus rhamnosus GG improves outcome in experimental pseudomonas aeruginosa pneumonia: potential role of regulatory T cells, Shock
Khailova, Frank, Dominguez, Wischmeyer, Probiotic administration reduces mortality and improves intestinal epithelial homeostasis in experimental sepsis, Anesthesiology
Khailova, Petrie, Baird, Rieg, Wischmeyer, Lactobacillus rhamnosus GG and Bifidobacterium longum attenuate lung injury and inflammatory response in experimental sepsis, PLoS One
King, Glanville, Sanders, Fitzgerald, Varley, Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis, The British journal of nutrition
Kiso, Takano, Sakabe, Protective efficacy of orally administered, heat-killed Lactobacillus pentosus b240 against influenza A virus, Sci Rep
Luoto, Ruuskanen, Waris, Kalliomäki, Salminen et al., Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: A randomized, placebo-controlled trial, J Allergy Clin Immunol
Manzanares, Lemieux, Langlois, Wischmeyer, Probiotic and synbiotic therapy in critical illness: a systematic review and meta-analysis, Crit Care
Mathieu, Ritchie, Ortiz-Ospina, A global database of COVID-19 vaccinations, Nature Human Behaviour
Morrow, Casale, Kollef, PROBIOTIC PROPHYLAXIS OF VENTILATOR-ASSOCIATED PNEUMONIA, CHEST
Morrow, Kollef, Casale, Probiotic prophylaxis of ventilator-associated pneumonia: a blinded, randomized, controlled trial, Am J Respir Crit Care Med
Padma, COVID vaccines to reach poorest countries in 2023 -despite recent pledges, Nature
Panigrahi, Parida, Nanda, A randomized synbiotic trial to prevent sepsis among infants in rural India, Nature
Stenman, Lehtinen, Meland, Probiotic With or Without Fiber Controls Body Fat Mass, Associated With Serum Zonulin, in Overweight and Obese Adults-Randomized Controlled Trial, EBioMedicine
Surana, Kasper, Moving beyond microbiome-wide associations to causal microbe identification, Nature
Tang, Bohannon, Lew, Randomised, double-blind, placebo-controlled trial of Probiotics To Eliminate COVID-19 Transmission in Exposed Household Contacts (PROTECT-EHC): a clinical trial protocol, BMJ Open
Walton, Gibson, Hunter, Mechanisms linking the human gut microbiome to prophylactic and treatment strategies for COVID-19, The British journal of nutrition
Wischmeyer, Mcdonald, Knight, Role of the microbiome, probiotics, and 'dysbiosis therapy' in critical illness, Curr Opin Crit Care
DOI record:
{
"DOI": "10.1101/2022.01.04.21268275",
"URL": "http://dx.doi.org/10.1101/2022.01.04.21268275",
"abstract": "<jats:title>STRUCTURED ABSTRACT</jats:title><jats:sec><jats:title>Importance</jats:title><jats:p>The COVID-19 pandemic continues to pose unprecedented challenges to worldwide health. While vaccines are effective, supplemental strategies to mitigate the spread and severity of COVID-19 are urgently needed. Emerging evidence suggests susceptibility to infections, including respiratory tract infections, may be reduced by probiotic interventions; therefore, probiotics may be a low-risk, widely implementable modality to mitigate risk of COVID-19 disease, particularly in areas with low vaccine availability and/or uptake.</jats:p></jats:sec><jats:sec><jats:title>Objective</jats:title><jats:p>To determine whether daily probiotic<jats:italic>Lactobacillus rhamnosus</jats:italic>GG (LGG) is effective in preventing development of symptoms of illness within 28 days of COVID-19 exposure.</jats:p></jats:sec><jats:sec><jats:title>Design</jats:title><jats:p>This randomized, double-blind, placebo-controlled trial across the United States (PROTECT-EHC) enrolled in 2020-2021. Participants were followed for 60 days.</jats:p></jats:sec><jats:sec><jats:title>Setting</jats:title><jats:p>Describe the study setting to assist readers to determine the applicability of the report to other circumstances, for example, multicenter, population-based, primary care or referral center(s), etc.</jats:p></jats:sec><jats:sec><jats:title>Participants</jats:title><jats:p>Participants included individuals ≥ 1 year of age with a household contact with a recent (≤ 7 days) diagnosis of COVID-19. 182 participants were enrolled and randomized during the study period.</jats:p></jats:sec><jats:sec><jats:title>Intervention</jats:title><jats:p>Participants were randomized to receive daily oral LGG or microcrystalline cellulose placebo for 28 days.</jats:p></jats:sec><jats:sec><jats:title>Main Outcomes and Measures</jats:title><jats:p>The primary outcome was development of symptoms within 28 days of exposure to a COVID-19-infected household contact. Stool was collected to evaluate for changes in microbiome structure.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>182 participants were enrolled and randomized during the study period. Intention-to-treat analysis showed that participants randomized to LGG were less likely to develop symptoms versus those randomized to placebo (26.4% vs. 42.9%, p=0.02). Further, LGG was associated with a statistically significant reduction in COVID-19 diagnosis (log rank p=0.049) via time-to-event analysis. Overall incidence of COVID-19 diagnosis did not significantly differ between LGG and placebo groups (8.8% vs. 15.4%, p=0.17). LGG was well-tolerated with no increased side effects versus placebo. Placebo recipients were more likely to stop the study product, temporarily or permanently, due to symptoms attributed to the study product (5.5% vs. 0%, p = 0.02).</jats:p></jats:sec><jats:sec><jats:title>Conclusions and Relevance</jats:title><jats:p>Our study suggests that LGG is well-tolerated and is associated with prolonged time to development of COVID-19 infection, reduced incidence of symptoms, and changes to gut microbiome structure when used as post-exposure prophylaxis within 7 days after exposure. This preliminary work may inform the approach to prevention of COVID-19, particularly in underdeveloped nations where<jats:italic>Lactobacillus</jats:italic>probiotics have already been utilized to reduce non-COVID sepsis and infectious-morbidity. This study was limited by its remote format, which necessitated a primary endpoint of self-reported symptoms rather than laboratory-confirmed infection; further laboratory-based studies are needed to further define the efficacy of LGG in preventing COVID-19 infection, especially in larger populations and including comparison of pre-exposure vs. post-exposure prophylaxis.</jats:p></jats:sec><jats:sec><jats:title>Trial registration</jats:title><jats:p><jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://clinicaltrials.gov/\">ClinicalTrials.gov</jats:ext-link>,<jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"clintrialgov\" xlink:href=\"NCT04399252\">NCT04399252</jats:ext-link>,<jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://clinicaltrials.gov/ct2/show/NCT04399252\">https://clinicaltrials.gov/ct2/show/NCT04399252</jats:ext-link></jats:p></jats:sec><jats:sec><jats:title>KEY POINTS</jats:title><jats:sec><jats:title>Question</jats:title><jats:p>Is daily probiotic<jats:italic>Lactobacillus rhamnosus</jats:italic>GG (LGG) effective in preventing development of symptoms of illness compatible with COVID-19 within 28 days of COVID-19 exposure compared to placebo?</jats:p></jats:sec><jats:sec><jats:title>Findings</jats:title><jats:p>In this randomized clinical trial that included 182 participants, the proportion who developed symptoms was 26.4% with LGG versus 42.9% with placebo, a significant difference.</jats:p></jats:sec><jats:sec><jats:title>Meaning</jats:title><jats:p>LGG probiotic may protect against the development of symptoms when used as post-exposure prophylaxis within 7 days after COVID-19 exposure.</jats:p></jats:sec></jats:sec>",
"accepted": {
"date-parts": [
[
2022,
1,
5
]
]
},
"author": [
{
"affiliation": [],
"family": "Wischmeyer",
"given": "Paul E.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Tang",
"given": "Helen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ren",
"given": "Yi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bohannon",
"given": "Lauren",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ramirez",
"given": "Zeni E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Andermann",
"given": "Tessa M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Messina",
"given": "Julia A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sung",
"given": "Julia A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jensen",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jung",
"given": "Sin-Ho",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Artica",
"given": "Alexandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Britt",
"given": "Anne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bush",
"given": "Amy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Johnson",
"given": "Ernaya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lew",
"given": "Meagan V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Miller",
"given": "Hilary M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pamanes",
"given": "Claudia E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Racioppi",
"given": "Alessandro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhao",
"given": "Aaron T.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9544-2551",
"affiliation": [],
"authenticated-orcid": false,
"family": "Surana",
"given": "Neeraj K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sung",
"given": "Anthony D.",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
1,
5
]
],
"date-time": "2022-01-05T19:30:14Z",
"timestamp": 1641411014000
},
"deposited": {
"date-parts": [
[
2023,
11,
15
]
],
"date-time": "2023-11-15T11:03:44Z",
"timestamp": 1700046224000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2024,
1,
5
]
],
"date-time": "2024-01-05T06:04:41Z",
"timestamp": 1704434681482
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 17,
"issued": {
"date-parts": [
[
2022,
1,
5
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2022.01.04.21268275",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
1,
5
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2022,
1,
5
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"key": "2022010703551062000_2022.01.04.21268275v1.1",
"unstructured": "WHO COVID-19 Dashboard. World Health Organization. https://covid19.who.int/. Updated 11/29/2021. Accessed 11/29/21."
},
{
"DOI": "10.1038/s41562-021-01122-8",
"article-title": "A global database of COVID-19 vaccinations",
"doi-asserted-by": "crossref",
"first-page": "947",
"issue": "7",
"journal-title": "Nature Human Behaviour",
"key": "2022010703551062000_2022.01.04.21268275v1.2",
"volume": "5",
"year": "2021"
},
{
"DOI": "10.1038/d41586-021-01762-w",
"article-title": "COVID vaccines to reach poorest countries in 2023 -despite recent pledges",
"doi-asserted-by": "crossref",
"first-page": "342",
"issue": "7867",
"journal-title": "Nature",
"key": "2022010703551062000_2022.01.04.21268275v1.3",
"volume": "595",
"year": "2021"
},
{
"DOI": "10.1038/d41586-021-02532-4",
"doi-asserted-by": "publisher",
"key": "2022010703551062000_2022.01.04.21268275v1.4"
},
{
"DOI": "10.1016/j.nut.2018.07.017",
"article-title": "Probiotic and synbiotic therapy in the critically ill: State of the art",
"doi-asserted-by": "crossref",
"first-page": "29",
"journal-title": "Nutrition",
"key": "2022010703551062000_2022.01.04.21268275v1.5",
"volume": "59",
"year": "2019"
},
{
"DOI": "10.1097/MCC.0000000000000321",
"doi-asserted-by": "publisher",
"key": "2022010703551062000_2022.01.04.21268275v1.6"
},
{
"DOI": "10.1017/S0007114514000075",
"doi-asserted-by": "publisher",
"key": "2022010703551062000_2022.01.04.21268275v1.7"
},
{
"DOI": "10.1038/nature23480",
"doi-asserted-by": "publisher",
"key": "2022010703551062000_2022.01.04.21268275v1.8"
},
{
"DOI": "10.1002/14651858.CD006895.pub3",
"doi-asserted-by": "crossref",
"key": "2022010703551062000_2022.01.04.21268275v1.9",
"unstructured": "Hao Q , Dong BR , Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database of Systematic Reviews. 2015(2)."
},
{
"DOI": "10.1164/rccm.200912-1853OC",
"doi-asserted-by": "publisher",
"key": "2022010703551062000_2022.01.04.21268275v1.10"
},
{
"DOI": "10.1186/s13054-016-1434-y",
"doi-asserted-by": "publisher",
"key": "2022010703551062000_2022.01.04.21268275v1.11"
},
{
"DOI": "10.1016/j.clnu.2016.09.025",
"doi-asserted-by": "publisher",
"key": "2022010703551062000_2022.01.04.21268275v1.12"
},
{
"DOI": "10.1097/SHK.0000000000000066",
"doi-asserted-by": "publisher",
"key": "2022010703551062000_2022.01.04.21268275v1.13"
},
{
"DOI": "10.1371/journal.pone.0097861",
"article-title": "Lactobacillus rhamnosus GG and Bifidobacterium longum attenuate lung injury and inflammatory response in experimental sepsis",
"doi-asserted-by": "crossref",
"first-page": "e97861",
"issue": "5",
"journal-title": "PLoS One",
"key": "2022010703551062000_2022.01.04.21268275v1.14",
"volume": "9",
"year": "2014"
},
{
"DOI": "10.1097/ALN.0b013e318291c2fc",
"doi-asserted-by": "publisher",
"key": "2022010703551062000_2022.01.04.21268275v1.15"
},
{
"DOI": "10.1038/srep01563",
"article-title": "Protective efficacy of orally administered, heat-killed Lactobacillus pentosus b240 against influenza A virus",
"doi-asserted-by": "crossref",
"first-page": "1563",
"journal-title": "Sci Rep",
"key": "2022010703551062000_2022.01.04.21268275v1.16",
"volume": "3",
"year": "2013"
},
{
"DOI": "10.1017/S0007114520003980",
"article-title": "Mechanisms linking the human gut microbiome to prophylactic and treatment strategies for COVID-19",
"doi-asserted-by": "crossref",
"first-page": "219",
"issue": "2",
"journal-title": "The British journal of nutrition",
"key": "2022010703551062000_2022.01.04.21268275v1.17",
"volume": "126",
"year": "2021"
},
{
"DOI": "10.1136/bmjopen-2020-047069",
"doi-asserted-by": "publisher",
"key": "2022010703551062000_2022.01.04.21268275v1.18"
},
{
"DOI": "10.1016/j.ebiom.2016.10.036",
"article-title": "Probiotic With or Without Fiber Controls Body Fat Mass, Associated With Serum Zonulin, in Overweight and Obese Adults-Randomized Controlled Trial",
"doi-asserted-by": "crossref",
"first-page": "190",
"journal-title": "EBioMedicine",
"key": "2022010703551062000_2022.01.04.21268275v1.19",
"volume": "13",
"year": "2016"
},
{
"DOI": "10.3920/BM2018.0028",
"article-title": "Probiotic or synbiotic alters the gut microbiota and metabolism in a randomised controlled trial of weight management in overweight adults",
"doi-asserted-by": "crossref",
"first-page": "121",
"issue": "2",
"journal-title": "Benef Microbes",
"key": "2022010703551062000_2022.01.04.21268275v1.20",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1016/j.jaci.2013.08.020",
"doi-asserted-by": "publisher",
"key": "2022010703551062000_2022.01.04.21268275v1.21"
},
{
"DOI": "10.1016/j.jbi.2008.08.010",
"doi-asserted-by": "publisher",
"key": "2022010703551062000_2022.01.04.21268275v1.22"
},
{
"DOI": "10.1038/nature25019",
"doi-asserted-by": "publisher",
"key": "2022010703551062000_2022.01.04.21268275v1.23"
},
{
"DOI": "10.1038/nmeth.3869",
"doi-asserted-by": "publisher",
"key": "2022010703551062000_2022.01.04.21268275v1.24"
},
{
"DOI": "10.15585/mmwr.mm6909e1",
"doi-asserted-by": "publisher",
"key": "2022010703551062000_2022.01.04.21268275v1.25"
},
{
"DOI": "10.1002/14651858.CD006895.pub3",
"doi-asserted-by": "crossref",
"key": "2022010703551062000_2022.01.04.21268275v1.26",
"unstructured": "Hao Q , Dong BR , Wu T. Probiotics for preventing acute upper respiratory tract infections. The Cochrane database of systematic reviews. 2015(2):CD006895."
},
{
"DOI": "10.1378/chest.136.4_MeetingAbstracts.36S-h",
"article-title": "PROBIOTIC PROPHYLAXIS OF VENTILATOR-ASSOCIATED PNEUMONIA",
"doi-asserted-by": "crossref",
"first-page": "36S",
"issue": "4",
"journal-title": "CHEST",
"key": "2022010703551062000_2022.01.04.21268275v1.27",
"volume": "136",
"year": "2009"
},
{
"DOI": "10.1002/ptr.7259",
"doi-asserted-by": "crossref",
"key": "2022010703551062000_2022.01.04.21268275v1.28",
"unstructured": "Farshbaf-Khalili A , Farajnia S , Pourzeinali S , Shakouri SK , Salehi-Pourmehr H. The effect of nanomicelle curcumin supplementation and Nigella sativa oil on the expression level of miRNA-21, miRNA-422a, and miRNA-503 gene in postmenopausal women with low bone mass density: A randomized, triple-blind, placebo-controlled clinical trial with factorial design. Phytother Res. 2021."
},
{
"DOI": "10.3390/nu11122913",
"doi-asserted-by": "crossref",
"key": "2022010703551062000_2022.01.04.21268275v1.29",
"unstructured": "Dore MP , Bibbò S , Fresi G , Bassotti G , Pes GM . Side Effects Associated with Probiotic Use in Adult Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2019;11(12)."
},
{
"DOI": "10.3390/nu9111175",
"doi-asserted-by": "crossref",
"key": "2022010703551062000_2022.01.04.21268275v1.30",
"unstructured": "Lei WT , Shih PC , Liu SJ , Lin CY , Yeh TL . Effect of Probiotics and Prebiotics on Immune Response to Influenza Vaccination in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2017;9(11)."
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2022.01.04.21268275"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Daily Lactobacillus Probiotic versus Placebo in COVID-19-Exposed Household Contacts (PROTECT-EHC): A Randomized Clinical Trial",
"type": "posted-content"
}
