Antiviral effectiveness and safety of azvudine in hospitalized SARS‐CoV‐2 patients with pre‐existing chronic respiratory diseases: A multicenter, retrospective cohort study
et al., VIEW, doi:10.1002/VIW.20240133, NCT06349655, Feb 2025
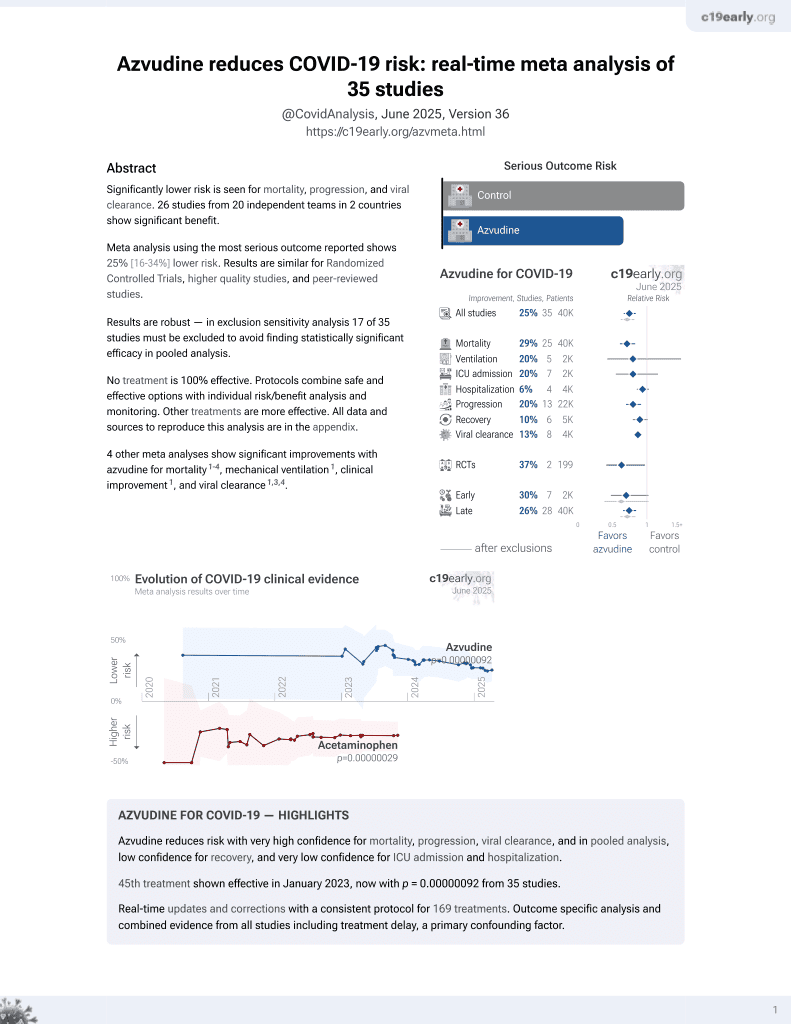
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.000000017 from 39 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
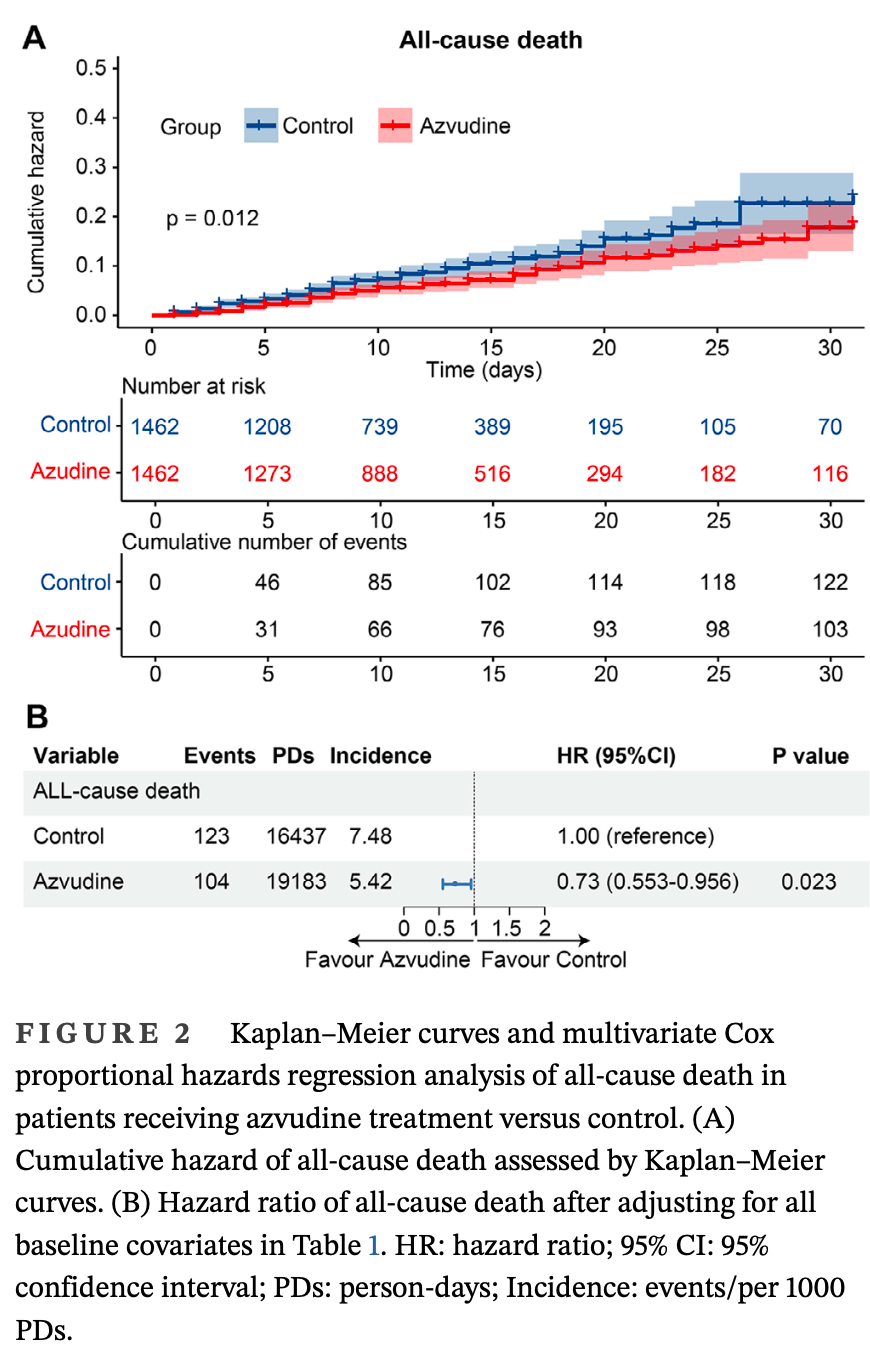
Retrospective 2,924 hospitalized COVID-19 patients with chronic respiratory diseases in China, showing lower all-cause mortality with azvudine, but no significant difference in composite disease progression.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments3.
|
risk of death, 27.0% lower, HR 0.73, p = 0.02, treatment 1,462, control 1,462, adjusted per study, propensity score matching, multivariable, Cox proportional hazards.
|
|
risk of progression, 15.0% higher, HR 1.15, p = 0.16, treatment 1,462, control 1,462, adjusted per study, progression to severe or death, propensity score matching, multivariable, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Xiong et al., Real-world data of Azvudine-induced hepatotoxicity among hospitalized COVID-19 patients in China: a retrospective case-control study, Frontiers in Pharmacology, doi:10.3389/fphar.2025.1558054.
Sun et al., 5 Feb 2025, retrospective, China, peer-reviewed, 18 authors, study period 5 December, 2022 - 31 January, 2023, trial NCT06349655 (history).
Contact: fccrenzg@zzu.edu.cn, fcccuigy@zzu.edu.cn.
Antiviral effectiveness and safety of azvudine in hospitalized SARS‐CoV‐2 patients with pre‐existing chronic respiratory diseases: A multicenter, retrospective cohort study
VIEW, doi:10.1002/viw.20240133
Although azvudine has become a priority in the treatment of SARS-CoV-2, its effectiveness and safety among COVID-19 patients who already have chronic respiratory diseases (CRDs) have not been sufficiently validated. A retrospective, multicenter cohort study involving 10 hospitals in Henan Province was
C O N F L I C T O F I N T E R E S T S TAT E M E N T The authors declare no conflict of interest.
D ATA AVA I L A B I L I T Y S TAT E M E N T All data relevant to the study are included in the article.
O R C I D Zhigang Ren https://orcid.org/0000-0003-0798-3444
R E F E R E N C E S
References
Cao, Ding, Xu, Li, Zheng et al., None, Front Med
Carr, Fajt, Kraft, Phipatanakul, Szefler et al., None, J Allergy Clin Immunol
Catania, Stati, Spitaleri, None, Tumori
Chen, Jiang, Rang, Zhuo, Zhou, None, Sci Rep
Chen, Lin, Lu, Wu, Pan et al., None, Diagn Microbiol Infect Dis
Chiner-Vives, Cordovilla-Pérez, De La Rosa-Carrillo, García-Clemente, Izquierdo-Alonso et al., None, Arch Bronconeumol
Christenson, Smith, Bafadhel, Putcha, None, Lancet
Dian, Meng, Sun, Deng, Zeng, None, J Infect
Dingemans, Soo, Jazieh, Rice, Kim et al., None, J Thorac Oncol
Faust, Kumar, Shah, Khadke, Dani et al., None, Clin Infect Dis
Graziani, Soriano, Del Rio-Bermudez, Morena, Díaz et al., None, J Clin Med
Herrera, Sanz, Shapira, Brotons, Chapple et al., None, J Clin Periodontol
Hyams, Qian, Nava, Challen, Begier et al., None, J R Soc Med
Kale, Shelke, Dagar, Anders, Gaikwad, None, Front Pharmacol
Liu, Rajeevan, Simonov, Lee, Wilson et al., None, J Allergy Clin Immunol Pract
Meng, Wei, Wu, Zeng, Luo et al., None, EClinicalMedicine
Miller, Cappuccio, None, Sleep Med Rev
Ren, Luo, Yu, Song, Liang et al., None, Adv Sci
Ren, Yang, Su, Qian, Yuan et al., None, J Infect
Shang, Li, Guo, Zhang, Wang, None, Front Pharmacol
Sheng, Li, Li, Wang, Wang et al., None, Acta Pharm Sin B
Sun, Jin, Dian, Shen, Zeng et al., None, EClinicalMedicine
Verma, Dhawan, Saied, Kaur, Kumar et al., None, Immun Inflamm Dis
Wei, Zeng, Wang, Gui, Zhang et al., None, Front Pharmacol
Wu, He, Huang, Guo, Li et al., None, Adv Sci
Yu, Chang, None, Innovation
Zhang, Li, Wang, Liu, Lu et al., None, Signal Transduct Target Ther
Zong, Zhou, Li, Jiang, Liu et al., None, Acta Pharm Sin B
DOI record:
{
"DOI": "10.1002/viw.20240133",
"ISSN": [
"2688-3988",
"2688-268X"
],
"URL": "http://dx.doi.org/10.1002/VIW.20240133",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Although azvudine has become a priority in the treatment of SARS‐CoV‐2, its effectiveness and safety among COVID‐19 patients who already have chronic respiratory diseases (CRDs) have not been sufficiently validated. A retrospective, multicenter cohort study involving 10 hospitals in Henan Province was performed to assess inpatients with COVID‐19 and CRDs (Clinical Trial Registration Number: NCT06349655). Azvudine recipients and the control group were matched at a 1:1 ratio using propensity scores. The clinical outcomes (all‐cause death and composite disease progression) were analyzed using Kaplan‒Meier and Cox regression analyses, with additional subgroup and sensitivity analyses performed. Eighteen clinical features were included to construct a nomogram for predicting the survival of inpatients with COVID‐19 and CRDs. Out of 37,606 hospitalized COVID‐19 patients, 1462 azvudine recipients and 1462 matched controls were included in the analysis. The results of Kaplan‒Meier and multivariate Cox regression analyses demonstrated that in contrast to the controls, azvudine use was associated with a decreased risk of all‐cause death in patients with COVID‐19 and pre‐existing CRDs (log‐rank: <jats:italic>p</jats:italic> = .012; HR: 0.73; 95% CI: 0.553‒0.956); but was not significantly different in terms of composite disease progression (log‐rank: <jats:italic>p</jats:italic> = .82; HR: 1.15; 95% CI: 0.948‒1.383). An analysis of subgroups and three sensitivity appraisals validate the above outcomes. The number and type of adverse events associated with azvudine treatment were acceptable. The concordance index (0.8499, 0.8497) and area under the curve (86.1%, 80.4%) of the nomogram showed satisfactory discriminative ability in the training and test sets. Azvudine could be effective in reducing all‐cause death among inpatients with COVID‐19 and CRDs and had few serious adverse events.</jats:p>",
"alternative-id": [
"10.1002/VIW.20240133"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2024-11-30"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2025-01-13"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2025-02-05"
}
],
"author": [
{
"affiliation": [
{
"name": "Department of Infectious Diseases State Key Laboratory of Antiviral Drugs Pingyuan Laboratory The First Affiliated Hospital of Zhengzhou University Zhengzhou China"
}
],
"family": "Sun",
"given": "Junyi",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases State Key Laboratory of Antiviral Drugs Pingyuan Laboratory The First Affiliated Hospital of Zhengzhou University Zhengzhou China"
}
],
"family": "Yang",
"given": "Mengzhao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Anesthesiology and Perioperative Medicine The Second Affiliated Hospital of Zhengzhou University Zhengzhou China"
}
],
"family": "Yao",
"given": "Daoke",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases State Key Laboratory of Antiviral Drugs Pingyuan Laboratory The First Affiliated Hospital of Zhengzhou University Zhengzhou China"
}
],
"family": "Zhou",
"given": "Yongjian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases State Key Laboratory of Antiviral Drugs Pingyuan Laboratory The First Affiliated Hospital of Zhengzhou University Zhengzhou China"
}
],
"family": "Hu",
"given": "Xiaobo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastrointestinal Surgery Nanyang Central Hospital Nanyang China"
}
],
"family": "Qian",
"given": "Guowu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Cardiovascular Medicine Henan Provincial Chest Hospital Affiliated to Zhengzhou University Zhengzhou China"
}
],
"family": "Yuan",
"given": "Yiqiang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory and Critical Care Medicine Fengqiu County People's Hospital Xinxiang China"
}
],
"family": "Li",
"given": "Silin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Guangshan County People's Hospital Guangshan County Xinyang China"
}
],
"family": "Luo",
"given": "Hong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases Shangqiu Municipal Hospital Shangqiu Henan Province China"
}
],
"family": "Zhang",
"given": "Shixi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Liver Disease The Affiliated Infectious Disease Hospital of Zhengzhou University Zhengzhou China"
}
],
"family": "Li",
"given": "Guangming",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases Anyang City Fifth People's Hospital Anyang China"
}
],
"family": "Zhang",
"given": "Donghua",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases Luoyang Central Hospital Affiliated to Zhengzhou University Luoyang China"
}
],
"family": "Li",
"given": "Guotao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Henan Center for Disease Control and Prevention Zhengzhou China"
}
],
"family": "Zhang",
"given": "Yanyang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases The First Affiliated Hospital, College of Clinical Medicine, Henan University of Science and Technology Luoyang China"
}
],
"family": "Hu",
"given": "Xinjun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases State Key Laboratory of Antiviral Drugs Pingyuan Laboratory The First Affiliated Hospital of Zhengzhou University Zhengzhou China"
}
],
"family": "Yu",
"given": "Zujiang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases State Key Laboratory of Antiviral Drugs Pingyuan Laboratory The First Affiliated Hospital of Zhengzhou University Zhengzhou China"
}
],
"family": "Cui",
"given": "Guangying",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-0798-3444",
"affiliation": [
{
"name": "Department of Infectious Diseases State Key Laboratory of Antiviral Drugs Pingyuan Laboratory The First Affiliated Hospital of Zhengzhou University Zhengzhou China"
}
],
"authenticated-orcid": false,
"family": "Ren",
"given": "Zhigang",
"sequence": "additional"
}
],
"container-title": "VIEW",
"container-title-short": "VIEW",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2025,
2,
6
]
],
"date-time": "2025-02-06T03:59:56Z",
"timestamp": 1738814396000
},
"deposited": {
"date-parts": [
[
2025,
2,
6
]
],
"date-time": "2025-02-06T04:00:02Z",
"timestamp": 1738814402000
},
"funder": [
{
"DOI": "10.13039/501100012166",
"award": [
"2023YFC3043514",
"2022YFC2303100"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100012166",
"id-type": "DOI"
}
],
"name": "National Key Research and Development Program of China"
}
],
"indexed": {
"date-parts": [
[
2025,
2,
6
]
],
"date-time": "2025-02-06T04:40:08Z",
"timestamp": 1738816808680,
"version": "3.37.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
2,
5
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
2,
5
]
],
"date-time": "2025-02-05T00:00:00Z",
"timestamp": 1738713600000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/VIW.20240133",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2025,
2,
5
]
]
},
"published-online": {
"date-parts": [
[
2025,
2,
5
]
]
},
"publisher": "Wiley",
"reference": [
{
"key": "e_1_2_10_2_1",
"unstructured": "WHO COVID‐19 dashboard https://covid19.who.int(accessed: December 2024)."
},
{
"DOI": "10.1016/j.eclinm.2024.102500",
"author": "Meng M.",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "e_1_2_10_3_1",
"volume": "69",
"year": "2024"
},
{
"DOI": "10.1177/01410768231184162",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_4_1"
},
{
"DOI": "10.1016/j.jaip.2023.07.006",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_5_1"
},
{
"DOI": "10.1016/S0140-6736(22)00470-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_6_1"
},
{
"DOI": "10.1016/j.arbres.2022.03.011",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_7_1"
},
{
"DOI": "10.3390/jcm10010009",
"author": "Graziani D.",
"doi-asserted-by": "crossref",
"first-page": "9",
"journal-title": "J Clin Med",
"key": "e_1_2_10_8_1",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1111/jcpe.13807",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_9_1"
},
{
"DOI": "10.1016/j.smrv.2020.101382",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_10_1"
},
{
"DOI": "10.1093/cid/ciad400",
"author": "Faust J. S.",
"doi-asserted-by": "crossref",
"first-page": "1257",
"journal-title": "Clin Infect Dis",
"key": "e_1_2_10_11_1",
"volume": "77",
"year": "2023"
},
{
"DOI": "10.1016/j.jaci.2022.12.800",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_12_1"
},
{
"author": "Yu B.",
"journal-title": "Innovation",
"key": "e_1_2_10_13_1",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1002/advs.202001435",
"author": "Ren Z.",
"doi-asserted-by": "crossref",
"journal-title": "Adv Sci",
"key": "e_1_2_10_14_1",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1038/s41392-021-00835-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_15_1"
},
{
"DOI": "10.1016/j.eclinm.2023.101981",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_16_1"
},
{
"DOI": "10.1016/j.apsb.2023.07.007",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_17_1"
},
{
"DOI": "10.1016/j.jinf.2024.106355",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_18_1"
},
{
"DOI": "10.1038/s41598-024-53862-y",
"author": "Chen M. P.",
"doi-asserted-by": "crossref",
"first-page": "3318",
"journal-title": "Sci Rep",
"key": "e_1_2_10_19_1",
"volume": "14",
"year": "2024"
},
{
"DOI": "10.3389/fphar.2023.1274294",
"author": "Wei A. H.",
"doi-asserted-by": "crossref",
"journal-title": "Front Pharmacol",
"key": "e_1_2_10_20_1",
"volume": "14",
"year": "2024"
},
{
"DOI": "10.3389/fphar.2024.1362345",
"author": "Shang N.",
"doi-asserted-by": "crossref",
"journal-title": "Front Pharmacol",
"key": "e_1_2_10_21_1",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.1016/j.jinf.2023.05.012",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_22_1"
},
{
"DOI": "10.1002/advs.202306050",
"author": "Wu L.",
"doi-asserted-by": "crossref",
"journal-title": "Adv Sci",
"key": "e_1_2_10_23_1",
"volume": "11",
"year": "2024"
},
{
"DOI": "10.3389/fphar.2023.1053814",
"author": "Kale A.",
"doi-asserted-by": "crossref",
"journal-title": "Front Pharmacol",
"key": "e_1_2_10_24_1",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1016/j.diagmicrobio.2024.116353",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_25_1"
},
{
"DOI": "10.1002/iid3.1020",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_26_1"
},
{
"DOI": "10.1177/0300891620951863",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_27_1"
},
{
"DOI": "10.1016/j.jtho.2020.05.001",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_28_1"
},
{
"DOI": "10.1016/j.apsb.2024.03.032",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_29_1"
},
{
"DOI": "10.1007/s11684-023-1037-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_30_1"
}
],
"reference-count": 29,
"references-count": 29,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/VIW.20240133"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Antiviral effectiveness and safety of azvudine in hospitalized SARS‐CoV‐2 patients with pre‐existing chronic respiratory diseases: A multicenter, retrospective cohort study",
"type": "journal-article",
"update-policy": "https://doi.org/10.1002/crossmark_policy"
}