Effects of Spirulina platensis Supplementation on COVID-19 Severity in Critically Ill Patients: A Randomized Clinical Trial
et al., Journal of Cellular and Molecular Anesthesia, doi:10.5812/jcma-149015, IRCT20200720048139N1, Sep 2024
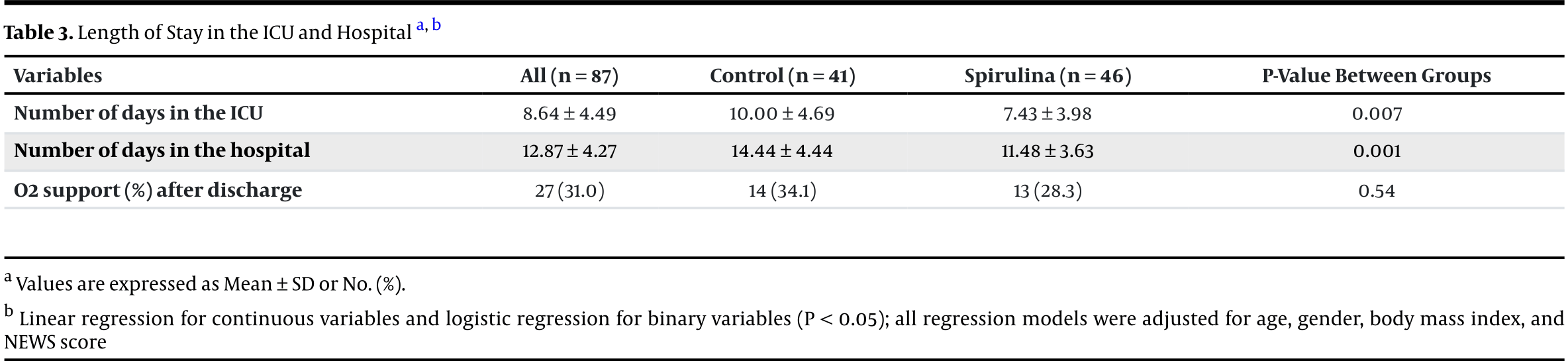
RCT 192 critically ill COVID-19 ICU patients showing reduced SOFA score, hospital stay and ICU stay with spirulina supplementation (5g/day), but no significant difference in mortality, NEWS2 score, APACHE score, NUTRIC score, or respiratory support at discharge.
|
risk of death, 3.0% higher, HR 1.03, p = 0.93, treatment 68, control 58, adjusted per study, multivariable, Cox proportional hazards.
|
|
ICU time, 25.7% lower, relative time 0.74, p = 0.007, treatment 46, control 41.
|
|
risk of oxygen therapy, 17.2% lower, RR 0.83, p = 0.64, treatment 13 of 46 (28.3%), control 14 of 41 (34.1%), NNT 17.
|
|
hospitalization time, 20.5% lower, relative time 0.80, p = 0.001, treatment 46, control 41.
|
|
relative NEWS reduction, 26.2% better, RR 0.74, p = 0.20, treatment mean 2.52 (±2.74) n=53, control mean 1.86 (±2.48) n=51.
|
|
relative APACHE reduction, 1.3% worse, RR 1.01, p = 0.97, treatment mean 2.39 (±3.58) n=53, control mean 2.42 (±4.35) n=51.
|
|
relative SOFA reduction, 10.8% better, RR 0.89, p = 0.62, treatment mean 1.67 (±1.72) n=53, control mean 1.49 (±1.94) n=51.
|
|
relative NUTRIC reduction, 23.0% better, RR 0.77, p = 0.37, treatment mean 1.13 (±1.61) n=53, control mean 0.87 (±1.33) n=51.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Shadnoush et al., 8 Sep 2024, Double Blind Randomized Controlled Trial, placebo-controlled, Iran, peer-reviewed, 15 authors, study period 1 March, 2021 - 6 December, 2021, trial IRCT20200720048139N1.
Contact: zeinabjavid93@gmail.com.
Effects of Spirulina platensis Supplementation on COVID-19 Severity in Critically Ill Patients: A Randomized Clinical Trial
Journal of Cellular and Molecular Anesthesia, doi:10.5812/jcma-149015
Background:Spirulina is a functional food with antioxidant and anti-inflammatory properties. Its anti-inflammatory, antioxidant, antiviral, and immunomodulatory potential may improve certain clinical conditions in patients with coronavirus disease 2019 in the intensive care unit (ICU). Objectives: This study aimed to investigate the effects of Spirulina supplementation on clinical conditions in critically ill patients with COVID-19. Methods: A double-blind clinical trial randomized patients admitted to an ICU to receive either Spirulina platensis supplementation (5g/day, n = 97) or to a control group (n = 95). The severity of COVID-19 was assessed using the Acute Physiology and Chronic Health Evaluation (APACHE), National Early Warning Score (NEWS) 2, and Sequential Organ Failure Assessment Score (SOFA). The study also evaluated the length of stay in the hospital and ICU, respiratory support at discharge, and 28-day mortality. In the survival analysis, 126 participants were evaluated (58 in the control group and 68 in the Spirulina group). Results: There was no significant difference between the groups in 28-day mortality (HR = 1.07, 95% CI 0.57 -1.97) or NEWS2 (P = 0.76). However, the SOFA score significantly decreased in the Spirulina group compared to the control group (P = 0.03). The Spirulina group had a shorter ICU length of stay (7.43 ± 3.98 days) compared to the control group (10.00 ± 4.69 days, P = 0.007). The hospital length of stay was also shorter in the Spirulina group compared to the control group (P = 0.001). Conclusions: Spirulina supplementation reduced disease severity (as measured by the SOFA score) and shortened hospital and ICU length of stay in critically ill COVID-19 patients, but it did not reduce mortality.
References
Ali, Saleh, Spirulina-an overview, International J Pharmacy Pharmaceutical Sci
Amazon, Spirulina herbal supplements
Arshad, Kilgore, Chaudhry, Jacobsen, Wang et al., Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19, Int J Infect Dis, doi:10.1016/j.ijid.2020.06.099
Barrón, Torres-Valencia, Chamorro-Cevallos, Zúñiga-Estrada, Spirulina as an antiviral agent
Belay, Spirulina, Arthrospira): production and quality assurance
Bhatt, Arora, Prajapati, Can algal derived bioactive metabolites serve as potential therapeutics for the treatment of SARS-CoV-2 like viral infection?, Front Microbiol, doi:10.3389/fmicb.2020.596374
Borchers, Belay, Keen, Gershwin, Spirulina and Immunity
Castillo, Costa, Barrios, Diaz, Miranda et al., Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study, J Steroid Biochem Mol Biol, doi:10.1016/j.jsbmb.2020.105751
Chamorro-Cevallos, Barrón, Toxicologic studies and antitoxic properties of Spirulina
Chen, Chang, Kuo, Huang, Hu et al., Welltolerated Spirulina extract inhibits influenza virus replication and reduces virus-induced mortality, Sci Rep, doi:10.1038/srep24253
Ciferri, Spirulina, the edible microorganism, Microbiol Rev, doi:10.1128/mr.47.4.551-578.1983
Cohen, The chemicals of Spirulina
Cyanotech, Spirulina®, None
Damiano, Sozio, Rosa, Santillo, NOX-dependent signaling dysregulation in severe COVID-19: Clues to effective treatments, Front Cell Infect Microbiol, doi:10.3389/fcimb.2020.608435
Dasta, Mclaughlin, Mody, Piech, Daily cost of an intensive care unit day: The contribution of mechanical ventilation, Crit Care Med, doi:10.1097/01.ccm.0000164543.14619.00
Febico, Biophyto® natural spirulina & chlorella
Ferreira, Polonini, Dijkers, Postulated adjuvant therapeutic strategies for COVID-19, J Pers Med, doi:10.3390/jpm10030080
Finamore, Palmery, Bensehaila, Peluso, Antioxidant, immunomodulating, and microbial-modulating activities of the sustainable and ecofriendly Spirulina, Oxid Med Cell Longev, doi:10.1155/2017/3247528
Garcia-Ruiz, Villalobos-Sanchez, Alam-Escamilla, Elizondo-Quiroga, In vitro inhibition of SARS-CoV-2 Infection by dry algae powders, Mol Anesth, doi:10.1038/s41598-022-22148-6
Gogna, Kaur, Sharma, Prasad, Singh et al., Spirulina-an edible cyanobacterium with potential therapeutic health benefits and toxicological consequences, J Am Nutr Assoc, doi:10.1080/27697061.2022.2103852
Grosshagauer, Kraemer, Somoza, The True Value of Spirulina, J Agric Food Chem, doi:10.1021/acs.jafc.9b08251
Gupta, Hayek, Chan, Mathews, Melamed, Factors associated with death in critically Ill patients with coronavirus disease 2019 in the US, JAMA Intern Med, doi:10.1001/jamainternmed.2020.3596
Gutierrez-Salmean, Fabila-Castillo, Chamorro-Cevallos, Nutritional and toxicological aspects of spirulina (Arthrospira), Nutr Hosp, doi:10.3305/nh.2015.32.1.9001
Habib, Parvin, Huntington, Hasan, A review on culture, production and use of Spirulina as food for humans and feeds for domestic animals
Hamedifard, Milajerdi, Reiner, Taghizadeh, Kolahdooz et al., The effects of spirulina on glycemic control and serum lipoproteins in patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials, Phytother Res, doi:10.1002/ptr.6441
Hatami, Ghalishourani, Najafgholizadeh, Pourmasoumi, Hadi et al., The effect of spirulina on type 2 diabetes: A systematic review and meta-analysis, J Diabetes Metab Disord, doi:10.1007/s40200-021-00760-z
Hatami, Mojani-Qomi, Javid, Taghavi, Bakhshandeh et al., Possible ameliorative role of Spirulina platensis on coagulation factors, lymphocytopenia, and malnutrition in ICU patients with COVID-19, Appl Physiol Nutr Metab, doi:10.1139/apnm-2022-0405
Hernandez Lepe, Wall-Medrano, Juarez-Oropeza, Ramos-Jimenez, Hernandez-Torres, Spirulina and its hypolipidemic and antioxidant effects in humans: A systematic review, Nutr Hosp, doi:10.3305/nh.2015.32.2.9100
Hojyo, Uchida, Tanaka, Hasebe, Tanaka et al., How COVID-19 induces cytokine storm with high mortality, Inflamm Regen, doi:10.1186/s41232-020-00146-3
Hosseini, Khosravi-Darani, Mozafari, Nutritional and medical applications of spirulina microalgae, Mini Rev Med Chem, doi:10.2174/1389557511313080009
Hu, Huang, Yin, The cytokine storm and COVID-19, J Med Virol, doi:10.1002/jmv.26232
Jones, Trzeciak, Kline, The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation, Crit Care Med, doi:10.1097/CCM.0b013e31819def97
Karkos, Leong, Karkos, Sivaji, Da, Spirulina in clinical practice: Evidence-based human applications, Evid Based Complement Alternat Med, doi:10.1093/ecam/nen058
Khan, Bhadouria, Bisen, Nutritional and therapeutic potential of Spirulina, Curr Pharm Biotechnol, doi:10.2174/138920105774370607
Krammer, Smith, Fouchier, Peiris, Kedzierska et al., None, Nat Rev Dis Primers, doi:10.1038/s41572-018-0002-y
Lambden, Laterre, Levy, Francois, The SOFA scoredevelopment, utility and challenges of accurate assessment in clinical trials, Crit Care, doi:10.1186/s13054-019-2663-7
Machowiec, Reka, Maksymowicz, Piecewicz-Szczesna, Smolen, Effect of Spirulina supplementation on systolic and diastolic blood pressure: Systematic review and meta-analysis of randomized controlled trials, Nutrients, doi:10.3390/nu13093054
Marles, Barrett, Barnes, Chavez, Gardiner et al., United States pharmacopeia safety evaluation of spirulina, Crit Rev Food Sci Nutr, doi:10.1080/10408391003721719
Mccarty, Clinical potential of Spirulina as a source of phycocyanobilin, J Med Food, doi:10.1089/jmf.2007.621
Menezes, Cumbers, Hogan, Arkin, Towards synthetic biological approaches to resource utilization on space missions, J R Soc Interface, doi:10.1098/rsif.2014.0715
Mohiti, Zarezadeh, Naeini, Tutunchi, Ostadrahimi et al., Spirulina supplementation and oxidative stress and pro-inflammatory biomarkers: A systematic review and metaanalysis of controlled clinical trials, Clin Exp Pharmacol Physiol, doi:10.1111/1440-1681.13510
Moradi, Ziaei, Foshati, Mohammadi, Nachvak et al., Effects of Spirulina supplementation on obesity: A systematic review and meta-analysis of randomized clinical trials, Complement Ther Med, doi:10.1016/j.ctim.2019.102211
Mubarakali, Mohamedsaalis, Sathya, Irfan, Kim, An evidence of microalgal peptides to target spike protein of COVID-19: In silico approach, Microb Pathog, doi:10.1016/j.micpath.2021.105189
Naeini, Zarezadeh, Mohiti, Tutunchi, Mamaghani et al., Spirulina supplementation as an adjuvant therapy in enhancement of antioxidant capacity: A systematic review and meta-analysis of controlled clinical trials, Int J Clin Pract, doi:10.1111/ijcp.14618
Nutritionals, Californian Spirulina
Penton-Rol, Marin-Prida, Mccarty, C-phycocyanin-derived phycocyanobilin as a potential nutraceutical approach for major neurodegenerative disorders and COVID-19-induced damage to the nervous system, Curr Neuropharmacol, doi:10.2174/1570159X19666210408123807
Pugh, Edwall, Lindmark, Kousoulas, Iyer et al., Oral administration of a Spirulina extract enriched for Braun-type lipoproteins protects mice against influenza A (H1N1) virus infection, Phytomedicine, doi:10.1016/j.phymed.2014.12.006
Raghunathan, Navabshan, Badar, Kim, Mubarakali, An investigation of algal peptides to target protein of lower respiratory tract infections: In silico approach, Biocatalysis Agricultural Biotechnol, doi:10.1016/j.bcab.2022.102585
Raschke, Agarwal, Rangan, Heise, Curry, Discriminant accuracy of the SOFA Score for determining the probable mortality of patients with COVID-19 Pneumonia requiring mechanical ventilation, JAMA, doi:10.1001/jama.2021.1545
Ratha, Renuka, Rawat, Bux, Prospective options of algaederived nutraceuticals as supplements to combat COVID-19 and human coronavirus diseases, Nutrition, doi:10.1016/j.nut.2020.111089
Ravi, De, Azharuddin, Paul, The beneficial effects of Spirulina focusing on its immunomodulatory and antioxidant properties, Nutrition and Dietary Supplements, doi:10.2147/NDS.S9838
Santos, Izidoro, Neutrophil-lymphocyte ratio in cardiovascular disease risk assessment, Int J Cardiovascular Sci, doi:10.5935/2359-4802.20180038
Santos, Silva, Translating the advanced glycation end products (AGEs) knowledge into real-world nutrition strategies, Eur J Clin Nutr, doi:10.1038/s41430-021-01028-8
Serban, Sahebkar, Dragan, Stoichescu-Hogea, Ursoniu et al., A systematic review and meta-analysis of the impact of Spirulina supplementation on plasma lipid concentrations, Clin Nutr, doi:10.1016/j.clnu.2015.09.007
Siedenburg, Cauchi, Spirulina (Arthrospira spp) as a complementary COVID-19 response option: Early evidence of promise, Current Res Nutrition Food Sci, doi:10.12944/CRNFSJ.10.1.10
Soni, Sudhakar, Rana, Spirulina-From growth to nutritional product: A review, Trends in food Sci Technol, doi:10.1016/j.tifs.2017.09.010
Tzachor, Rozen, Khatib, Jensen, Avni, Photosynthetically Controlled Spirulina, but Not Solar Spirulina, Inhibits TNF-alpha Secretion: Potential Implications for COVID-19-Related Cytokine Storm Therapy, Mar Biotechnol, doi:10.1007/s10126-021-10020-z
Vincent, De Mendonca, Cantraine, Moreno, Takala et al., Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine, Crit Care Med, doi:10.1097/00003246-199811000-00016
Wang, Zhang, Hou, Liu, Wang et al., The effects of pomegranate supplementation on biomarkers of inflammation and endothelial dysfunction: A meta-analysis and systematic review, Complement Ther Med, doi:10.1016/j.ctim.2020.102358
Yousefi, Saidpour, Mottaghi, The effects of Spirulina supplementation on metabolic syndrome components, its liver manifestation and related inflammatory markers: A systematic review, Complement Ther Med, doi:10.1016/j.ctim.2018.11.013
Zarezadeh, Faghfouri, Radkhah, Foroumandi, Khorshidi et al., Spirulina supplementation and anthropometric indices: A systematic review and meta-analysis of controlled clinical trials, Phytother Res, doi:10.1002/ptr.6834
DOI record:
{
"DOI": "10.5812/jcma-149015",
"ISSN": [
"2538-2462",
"2476-5120"
],
"URL": "http://dx.doi.org/10.5812/jcma-149015",
"abstract": "<jats:p>Background: Spirulina is a functional food with antioxidant and anti-inflammatory properties. Its anti-inflammatory, antioxidant, antiviral, and immunomodulatory potential may improve certain clinical conditions in patients with coronavirus disease 2019 (COVID-19) in the intensive care unit (ICU). Objectives: This study aimed to investigate the effects of Spirulina supplementation on clinical conditions in critically ill patients with COVID-19. Methods: A double-blind clinical trial randomized patients admitted to an ICU to receive either Spirulina platensis supplementation (5g/day, n = 97) or to a control group (n = 95). The severity of COVID-19 was assessed using the Acute Physiology and Chronic Health Evaluation (APACHE), National Early Warning Score (NEWS) 2, and Sequential Organ Failure Assessment Score (SOFA). The study also evaluated the length of stay in the hospital and ICU, respiratory support at discharge, and 28-day mortality. In the survival analysis, 126 participants were evaluated (58 in the control group and 68 in the Spirulina group). Results: There was no significant difference between the groups in 28-day mortality (HR = 1.07, 95% CI 0.57 - 1.97) or NEWS2 (P = 0.76). However, the SOFA score significantly decreased in the Spirulina group compared to the control group (P = 0.03). The Spirulina group had a shorter ICU length of stay (7.43 ± 3.98 days) compared to the control group (10.00 ± 4.69 days, P = 0.007). The hospital length of stay was also shorter in the Spirulina group compared to the control group (P = 0.001). Conclusions: Spirulina supplementation reduced disease severity (as measured by the SOFA score) and shortened hospital and ICU length of stay in critically ill COVID-19 patients, but it did not reduce mortality.</jats:p>",
"alternative-id": [
"daaa5bcacfa46a46835afa98319147a3ef506097"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"value": "2024-6-24"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Revised",
"name": "revised",
"order": 1,
"value": "2024-8-11"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2024-8-13"
},
{
"group": {
"label": "Import History",
"name": "import_history"
},
"label": "Import",
"name": "import",
"value": "Article is imported on 2024-09-08 12:16:02 by user ID: 221087."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-3716-0994",
"affiliation": [],
"authenticated-orcid": false,
"family": "Shadnoush",
"given": "Mahdi",
"sequence": "first"
},
{
"affiliation": [],
"family": "Santos",
"given": "Heitor O.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hatami",
"given": "Monireh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Norouzi",
"given": "Mehdi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Taghavi",
"given": "Mohsen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Santa Capita Cerqueira",
"given": "Henrique",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mojani- Qomi",
"given": "Mansoore Sadat",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5102-1501",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bakhshandeh",
"given": "Hooman",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rahmani",
"given": "Jamal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Javid",
"given": "Zainab",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sanaei Delir Zavaragh",
"given": "Davood",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mikaniki",
"given": "Fatemeh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chaharmahali",
"given": "Arezoo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ghanavati",
"given": "Matin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nazari",
"given": "Mojgan",
"sequence": "additional"
}
],
"container-title": "Journal of Cellular and Molecular Anesthesia",
"container-title-short": "J Cell Mol Anesth",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"brieflands.com"
]
},
"created": {
"date-parts": [
[
2024,
9,
8
]
],
"date-time": "2024-09-08T12:16:15Z",
"timestamp": 1725797775000
},
"deposited": {
"date-parts": [
[
2024,
11,
5
]
],
"date-time": "2024-11-05T22:04:01Z",
"timestamp": 1730844241000
},
"indexed": {
"date-parts": [
[
2024,
11,
5
]
],
"date-time": "2024-11-05T22:40:19Z",
"timestamp": 1730846419510,
"version": "3.28.0"
},
"is-referenced-by-count": 0,
"issue": "4",
"issued": {
"date-parts": [
[
2024,
9,
8
]
]
},
"journal-issue": {
"issue": "4",
"published-online": {
"date-parts": [
[
2024,
9,
8
]
]
}
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
8,
13
]
],
"date-time": "2024-08-13T00:00:00Z",
"timestamp": 1723507200000
}
}
],
"link": [
{
"URL": "https://brieflands.com/articles/jcma-149015",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://brieflands.com/articles/jcma-149015",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "3819",
"original-title": [],
"prefix": "10.5812",
"published": {
"date-parts": [
[
2024,
9,
8
]
]
},
"published-online": {
"date-parts": [
[
2024,
9,
8
]
]
},
"publisher": "Brieflands",
"reference": [
{
"DOI": "10.1128/mr.47.4.551-578.1983",
"doi-asserted-by": "publisher",
"key": "key-A149015REF1-1"
},
{
"DOI": "10.1080/10408391003721719",
"doi-asserted-by": "publisher",
"key": "key-A149015REF2-2"
},
{
"author": "Barrón BL",
"journal-title": "Spirulina as an antiviral agent.",
"key": "key-A149015REF3-3",
"year": "2008"
},
{
"author": "Belay A",
"journal-title": "Spirulina (Arthrospira): production and quality assurance.",
"key": "key-A149015REF4-4",
"year": "2008"
},
{
"author": "Cohen Z",
"journal-title": "The chemicals of Spirulina.",
"key": "key-A149015REF5-5",
"year": "1997"
},
{
"author": " ",
"journal-title": "LiverTox: Clinical and research information on drug-induced liver injury [Internet].",
"key": "key-A149015REF6-6",
"year": "2012"
},
{
"DOI": "10.3305/nh.2015.32.1.9001",
"doi-asserted-by": "publisher",
"key": "key-A149015REF7-7"
},
{
"author": "Chamorro-Cevallos G",
"journal-title": "Toxicologic studies and antitoxic properties of Spirulina.",
"key": "key-A149015REF8-8",
"year": "2007"
},
{
"DOI": "10.1093/ecam/nen058",
"doi-asserted-by": "publisher",
"key": "key-A149015REF9-9"
},
{
"DOI": "10.1080/27697061.2022.2103852",
"doi-asserted-by": "publisher",
"key": "key-A149015REF10-10"
},
{
"DOI": "10.1098/rsif.2014.0715",
"doi-asserted-by": "publisher",
"key": "key-A149015REF11-11"
},
{
"author": "Habib MAB",
"journal-title": "FAO Fisheries and Aquaculture Circular.",
"key": "key-A149015REF12-12",
"year": "2008"
},
{
"author": "Ali SK",
"first-page": "9",
"issue": "3",
"journal-title": "International J Pharmacy Pharmaceutical Sci.",
"key": "key-A149015REF13-13",
"volume": "4",
"year": "2012"
},
{
"DOI": "10.1021/acs.jafc.9b08251",
"doi-asserted-by": "publisher",
"key": "key-A149015REF14-14"
},
{
"DOI": "10.1016/j.tifs.2017.09.010",
"doi-asserted-by": "publisher",
"key": "key-A149015REF15-15"
},
{
"author": "Amazon ",
"journal-title": "Spirulina herbal supplements.",
"key": "key-A149015REF16-16",
"year": "2022"
},
{
"author": " ",
"journal-title": "Californian Spirulina.",
"key": "key-A149015REF17-17",
"year": "2022"
},
{
"author": "Cyanotech ",
"journal-title": "Hawaiian Spirulina®.",
"key": "key-A149015REF18-18",
"year": "2022"
},
{
"author": "FEBICO ",
"journal-title": "Biophyto® natural spirulina & chlorella.",
"key": "key-A149015REF19-19",
"year": "2022"
},
{
"DOI": "10.3305/nh.2015.32.2.9100",
"doi-asserted-by": "publisher",
"key": "key-A149015REF20-20"
},
{
"DOI": "10.1016/j.clnu.2015.09.007",
"doi-asserted-by": "publisher",
"key": "key-A149015REF21-21"
},
{
"DOI": "10.1016/j.ctim.2019.102211",
"doi-asserted-by": "publisher",
"key": "key-A149015REF22-22"
},
{
"DOI": "10.1002/ptr.6441",
"doi-asserted-by": "publisher",
"key": "key-A149015REF23-23"
},
{
"DOI": "10.1016/j.ctim.2018.11.013",
"doi-asserted-by": "publisher",
"key": "key-A149015REF24-24"
},
{
"DOI": "10.1007/s40200-021-00760-z",
"doi-asserted-by": "publisher",
"key": "key-A149015REF25-25"
},
{
"DOI": "10.1111/ijcp.14618",
"doi-asserted-by": "publisher",
"key": "key-A149015REF26-26"
},
{
"DOI": "10.1002/ptr.6834",
"doi-asserted-by": "publisher",
"key": "key-A149015REF27-27"
},
{
"DOI": "10.3390/nu13093054",
"doi-asserted-by": "publisher",
"key": "key-A149015REF28-28"
},
{
"DOI": "10.2174/138920105774370607",
"doi-asserted-by": "publisher",
"key": "key-A149015REF29-29"
},
{
"DOI": "10.2147/NDS.S9838",
"doi-asserted-by": "publisher",
"key": "key-A149015REF30-30"
},
{
"DOI": "10.2174/1389557511313080009",
"doi-asserted-by": "publisher",
"key": "key-A149015REF31-31"
},
{
"DOI": "10.1038/s41572-018-0002-y",
"doi-asserted-by": "publisher",
"key": "key-A149015REF32-32"
},
{
"DOI": "10.1016/j.phymed.2014.12.006",
"doi-asserted-by": "publisher",
"key": "key-A149015REF33-33"
},
{
"DOI": "10.1038/srep24253",
"doi-asserted-by": "publisher",
"key": "key-A149015REF34-34"
},
{
"author": "Borchers AT",
"journal-title": "Gershwin ME. Spirulina and Immunity.",
"key": "key-A149015REF35-35",
"year": "2007"
},
{
"DOI": "10.1016/j.micpath.2021.105189",
"doi-asserted-by": "publisher",
"key": "key-A149015REF36-36"
},
{
"DOI": "10.1016/j.bcab.2022.102585",
"doi-asserted-by": "publisher",
"key": "key-A149015REF37-37"
},
{
"DOI": "10.12944/CRNFSJ.10.1.10",
"doi-asserted-by": "publisher",
"key": "key-A149015REF38-38"
},
{
"DOI": "10.1038/s41598-022-22148-6",
"doi-asserted-by": "publisher",
"key": "key-A149015REF39-39"
},
{
"DOI": "10.1007/s10126-021-10020-z",
"doi-asserted-by": "publisher",
"key": "key-A149015REF40-40"
},
{
"DOI": "10.3389/fmicb.2020.596374",
"doi-asserted-by": "publisher",
"key": "key-A149015REF41-41"
},
{
"DOI": "10.1139/apnm-2022-0405",
"doi-asserted-by": "publisher",
"key": "key-A149015REF42-42"
},
{
"DOI": "10.1097/00003246-199811000-00016",
"doi-asserted-by": "publisher",
"key": "key-A149015REF43-43"
},
{
"DOI": "10.1097/CCM.0b013e31819def97",
"doi-asserted-by": "publisher",
"key": "key-A149015REF44-44"
},
{
"DOI": "10.1016/j.nut.2020.111089",
"doi-asserted-by": "publisher",
"key": "key-A149015REF45-45"
},
{
"DOI": "10.1089/jmf.2007.621",
"doi-asserted-by": "publisher",
"key": "key-A149015REF46-46"
},
{
"DOI": "10.2174/1570159X19666210408123807",
"doi-asserted-by": "publisher",
"key": "key-A149015REF47-47"
},
{
"DOI": "10.3389/fcimb.2020.608435",
"doi-asserted-by": "publisher",
"key": "key-A149015REF48-48"
},
{
"DOI": "10.1186/s41232-020-00146-3",
"doi-asserted-by": "publisher",
"key": "key-A149015REF49-49"
},
{
"DOI": "10.1002/jmv.26232",
"doi-asserted-by": "publisher",
"key": "key-A149015REF50-50"
},
{
"DOI": "10.1155/2017/3247528",
"doi-asserted-by": "publisher",
"key": "key-A149015REF51-51"
},
{
"DOI": "10.3390/jpm10030080",
"doi-asserted-by": "publisher",
"key": "key-A149015REF52-52"
},
{
"DOI": "10.1111/1440-1681.13510",
"doi-asserted-by": "publisher",
"key": "key-A149015REF53-53"
},
{
"DOI": "10.1186/s13054-019-2663-7",
"doi-asserted-by": "publisher",
"key": "key-A149015REF54-54"
},
{
"DOI": "10.1001/jama.2021.1545",
"doi-asserted-by": "publisher",
"key": "key-A149015REF55-55"
},
{
"DOI": "10.1001/jamainternmed.2020.3596",
"doi-asserted-by": "publisher",
"key": "key-A149015REF56-56"
},
{
"DOI": "10.1097/01.ccm.0000164543.14619.00",
"doi-asserted-by": "publisher",
"key": "key-A149015REF57-57"
},
{
"DOI": "10.1016/j.ctim.2020.102358",
"doi-asserted-by": "publisher",
"key": "key-A149015REF58-58"
},
{
"DOI": "10.1038/s41430-021-01028-8",
"doi-asserted-by": "publisher",
"key": "key-A149015REF59-59"
},
{
"DOI": "10.5935/2359-4802.20180038",
"doi-asserted-by": "publisher",
"key": "key-A149015REF60-60"
},
{
"DOI": "10.1016/j.jsbmb.2020.105751",
"doi-asserted-by": "publisher",
"key": "key-A149015REF61-61"
},
{
"DOI": "10.1016/j.ijid.2020.06.099",
"doi-asserted-by": "publisher",
"key": "key-A149015REF62-62"
}
],
"reference-count": 62,
"references-count": 62,
"relation": {},
"resource": {
"primary": {
"URL": "https://brieflands.com/articles/jcma-149015"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Effects of Spirulina platensis Supplementation on COVID-19 Severity in Critically Ill Patients: A Randomized Clinical Trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.5812/crossmark_update_policy",
"volume": "9"
}
shadnoush