Pentoxifylline Effects on Hospitalized COVID-19 Patients with Cytokine Storm Syndrome: A Randomized Clinical Trial
et al., Pharmaceuticals, doi:10.3390/ph16040631, NCT04739345, Apr 2023
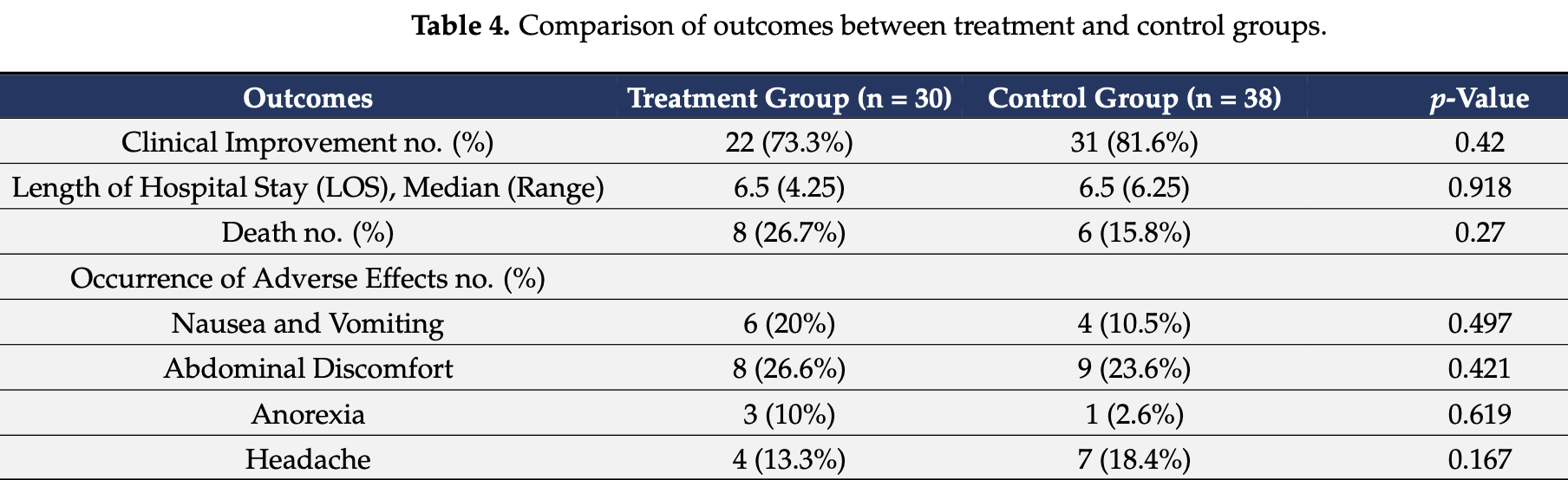
RCT 68 hospitalized COVID-19 patients with cytokine storm showing no significant improvement in clinical outcomes with pentoxifylline (PTX) treatment compared to standard therapy. While PTX demonstrated positive effects on inflammatory biomarkers like C-reactive protein and interleukin-6, there was no statistical difference in clinical improvement, hospital length of stay, or mortality.
|
risk of death, 68.9% higher, RR 1.69, p = 0.37, treatment 8 of 30 (26.7%), control 6 of 38 (15.8%).
|
|
risk of no improvement, 44.8% higher, RR 1.45, p = 0.56, treatment 8 of 30 (26.7%), control 7 of 38 (18.4%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Sarhan et al., 21 Apr 2023, Randomized Controlled Trial, Egypt, peer-reviewed, 9 authors, study period November 2020 - April 2021, trial NCT04739345 (history).
Contact: aealtyar@kau.edu.sa (corresponding author), raniamohammad87@yahoo.com, mariansobhy31@yahoo.com, ahmedessamabouwarda@gmail.com, yasmine.m.saied@gmail.com, drhaythamsoliman@gmail.com, nevine.mohamed@miuegypt.edu.eg, mona.schaalan@miuegypt.edu.eg, shaimaa.fathy@miuegypt.edu.eg.
Pentoxifylline Effects on Hospitalized COVID-19 Patients with Cytokine Storm Syndrome: A Randomized Clinical Trial
Pharmaceuticals, doi:10.3390/ph16040631
COVID-19 is a fatal, fast-spreading pandemic, and numerous attempts are being made around the world to understand and manage the disease. COVID-19 patients may develop a cytokinerelease syndrome, which causes serious respiratory diseases and, in many cases, death. The study examined the feasibility of employing legally available anti-inflammatory pentoxifylline (PTX), a low toxicity and cost medication, to mitigate the hyper-inflammation caused by COVID-19. Thirty adult patients who tested positive for SARS-CoV2 were hospitalized owing to the cytokine storm syndrome. They were given 400 mg of pentoxifylline orally TID according to the standard COVID-19 protocol of the Egyptian Ministry of Health. Besides this, a group of thirty-eight hospitalized COVID-19 patients who received the standard COVID-19 protocol was included in the study as a control group. The outcomes included laboratory test parameters, clinical improvements, and number of deaths in both groups. After receiving PTX, all patients showed a significant improvement in C reactive protein (CRP), and interleukin-6 (IL-6) levels at p < 0.01 and p = 0.004, respectively, while there was an increase in total leukocyte count (TLC) and neutrophil-to-leucocyte ratio (NLR) at p < 0.01 compared to their baseline levels. The D-dimer level showed a significant increase in the treatment group at p < 0.01, while showing no statistically significant difference in the control group. The median initial ALT (42 U/L) in the treatment group showed a decrease compared to the control group (51 U/L). No statistical significance was reported regarding clinical improvement, length of stay, and death percentages between the two groups. Our results showed no significant improvement of PTX over controls in clinical outcomes of hospitalized COVID-19 patients. Nevertheless, PTX displayed a positive effect on certain inflammatory biomarkers.
Informed Consent Statement: Written informed consent was obtained from all the participants in the study.
References
Azizi, Rouhani, Shaki, Karimpour-Razkenari, Ghazaeian et al., Pentoxifylline effects on hospitalized patients with COVID19: A randomized, double-blind clinical trial, Int. Immunopharmacol, doi:10.1016/j.intimp.2021.108227
Brie, Sahebkar, Penson, Dinca, Ursoniu et al., Effects of pentoxifylline on inflammatory markers and blood pressure: A systematic review and meta-analysis of randomized controlled trials, J. Hypertens, doi:10.1097/HJH.0000000000001086
Champion, Lapidus, Cherié, Spagnoli, Oliary et al., Pentoxifylline in heart failure: A meta-analysis of clinical trials, Cardiovasc. Ther, doi:10.1111/1755-5922.12076
Chavarría, Vázquez, Cherit, Bello, Suastegui et al., Antioxidants and pentoxifylline as coadjuvant measures to standard therapy to improve prognosis of patients with pneumonia by COVID-19, Comput. Struct. Biotechnol. J, doi:10.1016/j.csbj.2021.02.009
Chousterman, Swirski, Weber, Cytokine storm and sepsis disease pathogenesis
Dhameliya, Thakkar, Trivedi, Mesara, Subramanian, Pentoxifylline: An immunomodulatory drug for the treatment of COVID-19, J. Pure Appl. Microbiol, doi:10.22207/JPAM.14.SPL1.23
Dinicolantonio, Barroso-Aranda, Harnessing adenosine A2A receptors as a strategy for suppressing the lung inflammation and thrombotic complications of COVID-19: Potential of pentoxifylline and dipyridamole, Med. Hypotheses, doi:10.1016/j.mehy.2020.110051
Dong, Yuan, Xie, Pentoxifylline exerts anti-inflammatory effects on cerebral ischemia reperfusion-induced injury in a rat model via the p38 mitogen-activated protein kinase signaling pathway, Mol. Med. Rep, doi:10.3892/mmr.2017.7953
Feret, Nalewajska, Wojczy Ński, Witkiewicz, Kłos et al., Pentoxifylline as a Potential Adjuvant Therapy for COVID-19: Impeding the Burden of the Cytokine Storm, J. Clin. Med, doi:10.3390/jcm10225305
Gao, Xu, Wang, Liu, Cytokine storm syndrome in coronavirus disease 2019: A narrative review, J. Intern. Med, doi:10.1111/joim.13144
Ghasemnejad-Berenji, Pashapour, Sadeghpour, Pentoxifylline: A drug with antiviral and anti-inflammatory effects to be considered in the treatment of coronavirus disease 2019, Med. Princ. Pract, doi:10.1159/000512234
Giorgi, Cardarelli, Ragusa, Saliola, Biagioni et al., Phosphodiesterase Inhibitors: Could They Be Beneficial for the Treatment of COVID-19?, Int. J. Mol. Sci, doi:10.3390/ijms21155338
González-Pacheco, Amezcua-Guerra, Sandoval, Arias-Mendoza, Hypothesis for potential pathogenesis of SARS-CoV-2 infection-A review of immune changes in patients with viral pneumonia, Emerg. Microbes Infect
González-Pacheco, Amezcua-Guerra, Sandoval, Arias-Mendoza, Potential usefulness of pentoxifylline, a nonspecific phosphodiesterase inhibitor with anti-inflammatory, anti-thrombotic, antioxidant, and anti-fibrogenic properties, in the treatment of SARS-CoV-2, Eur. Rev. Med. Pharmacol. Sci
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Lai, Ko, Lee, Jean, Hsueh, Extra-respiratory manifestations of COVID-19, Int. J. Antimicrob. Agents, doi:10.1016/j.ijantimicag.2020.106024
Lee, Gardner, Porter, Louis, Ahmed et al., Current concepts in the diagnosis and management of cytokine release syndrome, Blood J. Am. Soc. Hematol, doi:10.1182/blood-2014-05-552729
Li, Liu, Mao, Xiao, Wang et al., Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: A systematic review and meta-analysis, Crit. Care, doi:10.1186/s13054-020-03374-8
Li, Zuo, Tang, Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases, Front. Pharmacol, doi:10.3389/fphar.2018.01048
Mahajan, Acute lung injury: Options to improve oxygenation, Contin. Educ. Anaesth. Crit. Care Pain, doi:10.1093/bjaceaccp/mki013
Maldonado, Hernandez-Ramírez, Oliva-Pérez, Sánchez-Martínez, Pimentel-González et al., Pentoxifylline decreases serum LDH levels and increases lymphocyte count in COVID-19 patients: Results from an external pilot study, Int. Immunopharmacol, doi:10.1016/j.intimp.2020.107209
Maldonado, Loza-Mejía, Chávez-Alderete, Repositioning of pentoxifylline as an immunomodulator and regulator of the renin-angiotensin system in the treatment of COVID-19, Med. Hypotheses, doi:10.1016/j.mehy.2020.109988
Marc, Moldovan, Hoza, Magheru, Ciavoi et al., Comparative Study of Cytokine Storm Treatment in Patients with COVID-19 Pneumonia Using Im-munomodulators, J. Clin. Med, doi:10.3390/jcm11102945
Marques, Zheng, Poulakis, Guzman, Costabel, Pentoxifylline inhibits TNF-α production from human alveolar macrophages, Am. J. Respir. Crit. Care Med, doi:10.1164/ajrccm.159.2.9804085
Martin, Jimenez, Mueóz-Fernández, Pentoxifylline and severe acute respiratory syndrome (SARS): A drug to be considered, Med. Sci. Monit
Matthay, Zemans, Zimmerman, Arabi, Beitler et al., Acute respiratory distress syndrome, Nat. Rev. Dis. Prim, doi:10.1038/s41572-019-0069-0
Mehta, Fajgenbaum, Is severe COVID-19 a cytokine storm syndrome: A hyperinflammatory debate, Curr. Opin. Rheumatol, doi:10.1097/BOR.0000000000000822
Michetti, Coimbra, Hoyt, Loomis, Junger et al., Pentoxifylline reduces acute lung injury in chronic endotoxemia, J. Surg. Res, doi:10.1016/S0022-4804(03)00219-1
Milne, Palmer, Anti-inflammatory and immunosuppressive effects of the A2A adenosine receptor, Sci. World J, doi:10.1100/tsw.2011.22
Monji, Siddiquee, Hashemian, Potential Role of Methylxanthines as an Adjuvant to COVID-19 Treatment: A Review of Pentoxifylline and Caffeine as the Case of Any Port in the Storm, Authorea Preprints, doi:10.22541/au.159015270.02586731
Montero, Martínez-Barrio, Serrano-Benavente, González, Rivera et al., Coronavirus disease 2019 (COVID-19) in autoimmune and inflammatory conditions: Clinical characteristics of poor outcomes, Rheumatol. Int, doi:10.1007/s00296-020-04676-4
Navarro, Punzón, Jiménez, Fernández-Cruz, Pizarro et al., Inhibition of Phosphodiesterase Type IV Suppresses Human Immunodeficiency Virus Type 1 Replication and Cytokine Production in Primary T Cells: Involvement of NF-κB and NFAT, J. Virol, doi:10.1128/JVI.72.6.4712-4720.1998
Palladino, Complete blood count alterations in COVID-19 patients: A narrative review, Biochem. Med, doi:10.11613/BM.2021.030501
Pammi, Haque, Pentoxifylline for treatment of sepsis and necrotizing enterocolitis in neonates, Cochrane Database Syst. Rev, doi:10.1002/14651858.CD004205.pub3
Parker, Armstrong, Corbett, Rowe, Houlihan, Systematic review: Pentoxifylline for the treatment of severe alcoholic hepatitis, Aliment. Pharmacol. Ther, doi:10.1111/apt.12279
Ramonfaur, González-Assad, Paredes-Vázquez, Pentoxifylline and COVID-19: A Systematic Review, doi:10.1101/2020.09.14.20194381
Salton, Confalonieri, Campisciano, Cifaldi, Rizzardi et al., Cytokine Profiles as Potential Prognostic and Therapeutic Markers in SARS-CoV-2-Induced ARDS, J. Clin. Med, doi:10.3390/jcm11112951
Sarhan, Harb, Warda, Salem-Bekhit, Shakeel et al., Efficacy of the early treatment with tocilizumab-hydroxychloroquine and tocilizumab-remdesivir in severe COVID-19 Patients, J. Infect. Public Health, doi:10.1016/j.jiph.2021.10.024
Sayah, Berkane, Guermache, Sabri, Lakhal et al., Interleukin-6, procalcitonin and neutrophil-to-lymphocyte ratio: Potential immune-inflammatory parameters to identify severe and fatal forms of COVID-19, Cytokine, doi:10.1016/j.cyto.2021.155428
Seirafianpour, Mozafarpoor, Fattahi, Sadeghzadeh-Bazargan, Hanifiha et al., Treatment of COVID-19 with pentoxifylline: Could it be a potential adjuvant therapy?, Dermatol. Ther, doi:10.1111/dth.13733
Selim, Leukocyte count in COVID-19: An important consideration, J. Bronchol, doi:10.1186/s43168-020-00045-8
Serdar, Cihan, Yücel, Serdar, Sample size, power and effect size revisited: Simplified and practical approaches in pre-clinical, clinical and laboratory studies, Biochem. Med, doi:10.11613/BM.2021.010502
Smilowitz, Kunichoff, Garshick, Shah, Pillinger et al., C-reactive protein and clinical outcomes in patients with COVID-19, Eur. Heart J, doi:10.1093/eurheartj/ehaa1103
Staubach, Schröder, Stüber, Gehrke, Traumann et al., Effect of pentoxifylline in severe sepsis: Results of a randomized, double-blind, placebo-controlled study, Arch. Surg, doi:10.1001/archsurg.133.1.94
Tay, Poh, Rénia, Macary, Ng, The trinity of COVID-19: Immunity, inflammation and intervention, Nat. Rev. Immunol, doi:10.1038/s41577-020-0311-8
Viechtbauer, Smits, Kotz, Budé, Spigt et al., A simple formula for the calculation of sample size in pilot studies, J. Clin. Epidemiol, doi:10.1016/j.jclinepi.2015.04.014
Violetis, Chasouraki, Giannou, Baraboutis, COVID-19 infection and haematological involvement: A review of epidemiology, pathophysiology and prognosis of full blood count findings, SN Compr. Clin. Med, doi:10.1007/s42399-020-00380-3
Wen, Lee, Siang, Koh, Repurposing pentoxifylline for the treatment of fibrosis: An overview, Adv. Ther, doi:10.1007/s12325-017-0547-2
Yang, Duan, Pan, Xu, Liu et al., Preventive effect of pentoxifylline on contrast-induced acute kidney injury in hypercholesterolemic rats, Exp. Ther. Med, doi:10.3892/etm.2014.2132
Yao, Cao, Wang, Shi, Liu et al., D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: A case control study, J. Intensive Care, doi:10.1186/s40560-020-00466-z
Zanza, Romenskaya, Manetti, Franceschi, La Russa et al., Cytokine Storm in COVID-19: Immunopathogenesis and Therapy, Medicina, doi:10.3390/medicina58020144
DOI record:
{
"DOI": "10.3390/ph16040631",
"ISSN": [
"1424-8247"
],
"URL": "http://dx.doi.org/10.3390/ph16040631",
"abstract": "<jats:p>COVID-19 is a fatal, fast-spreading pandemic, and numerous attempts are being made around the world to understand and manage the disease. COVID-19 patients may develop a cytokine-release syndrome, which causes serious respiratory diseases and, in many cases, death. The study examined the feasibility of employing legally available anti-inflammatory pentoxifylline (PTX), a low toxicity and cost medication, to mitigate the hyper-inflammation caused by COVID-19. Thirty adult patients who tested positive for SARS-CoV2 were hospitalized owing to the cytokine storm syndrome. They were given 400 mg of pentoxifylline orally TID according to the standard COVID-19 protocol of the Egyptian Ministry of Health. Besides this, a group of thirty-eight hospitalized COVID-19 patients who received the standard COVID-19 protocol was included in the study as a control group. The outcomes included laboratory test parameters, clinical improvements, and number of deaths in both groups. After receiving PTX, all patients showed a significant improvement in C reactive protein (CRP), and interleukin-6 (IL-6) levels at p < 0.01 and p = 0.004, respectively, while there was an increase in total leukocyte count (TLC) and neutrophil-to-leucocyte ratio (NLR) at p < 0.01 compared to their baseline levels. The D-dimer level showed a significant increase in the treatment group at p < 0.01, while showing no statistically significant difference in the control group. The median initial ALT (42 U/L) in the treatment group showed a decrease compared to the control group (51 U/L). No statistical significance was reported regarding clinical improvement, length of stay, and death percentages between the two groups. Our results showed no significant improvement of PTX over controls in clinical outcomes of hospitalized COVID-19 patients. Nevertheless, PTX displayed a positive effect on certain inflammatory biomarkers.</jats:p>",
"alternative-id": [
"ph16040631"
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-0781-6454",
"affiliation": [
{
"name": "Clinical Pharmacy Department, Faculty of Pharmacy, Beni-Suef University, Beni-Suef 62511, Egypt"
}
],
"authenticated-orcid": false,
"family": "Sarhan",
"given": "Rania M.",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0001-6210-3628",
"affiliation": [
{
"name": "Department of Pharmacy Practice, Faculty of Pharmacy, King Abdulaziz University, P.O. Box 80260, Jeddah 21589, Saudi Arabia"
},
{
"name": "Pharmacy Program, Batterjee Medical College, P.O. Box 6231, Jeddah 21442, Saudi Arabia"
}
],
"authenticated-orcid": false,
"family": "E. Altyar",
"given": "Ahmed",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-4434-6217",
"affiliation": [
{
"name": "Clinical Pharmacy Department, Faculty of Pharmacy, October 6 University, Giza 12585, Egypt"
}
],
"authenticated-orcid": false,
"family": "Essam Abou Warda",
"given": "Ahmed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Microbiology and Immunology Postgraduate Program, Faculty of Pharmacy, Cairo University, Cairo 11828, Egypt"
}
],
"family": "Saied",
"given": "Yasmine Mohamed",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-3584-1953",
"affiliation": [
{
"name": "Cardiology Department, Faculty of Medicine, El-Fayoum University, El-Fayoum 63514, Egypt"
}
],
"authenticated-orcid": false,
"family": "Ibrahim",
"given": "Haytham Soliman Ghareeb",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-8569-689X",
"affiliation": [
{
"name": "Clinical Pharmacy Department, Faculty of Pharmacy, Misr International University, Cairo 11828, Egypt"
}
],
"authenticated-orcid": false,
"family": "Schaalan",
"given": "Mona F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Pharmacy Department, Faculty of Pharmacy, Misr International University, Cairo 11828, Egypt"
}
],
"family": "Fathy",
"given": "Shaimaa",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-4067-5518",
"affiliation": [
{
"name": "Clinical Pharmacy Department, Faculty of Pharmacy, Misr International University, Cairo 11828, Egypt"
}
],
"authenticated-orcid": false,
"family": "Sarhan",
"given": "Neven",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-4916-4359",
"affiliation": [
{
"name": "Clinical Pharmacy Department, Faculty of Pharmacy, Beni-Suef University, Beni-Suef 62511, Egypt"
}
],
"authenticated-orcid": false,
"family": "Boshra",
"given": "Marian S.",
"sequence": "additional"
}
],
"container-title": "Pharmaceuticals",
"container-title-short": "Pharmaceuticals",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
4,
21
]
],
"date-time": "2023-04-21T11:51:17Z",
"timestamp": 1682077877000
},
"deposited": {
"date-parts": [
[
2023,
4,
21
]
],
"date-time": "2023-04-21T12:01:55Z",
"timestamp": 1682078515000
},
"indexed": {
"date-parts": [
[
2025,
2,
21
]
],
"date-time": "2025-02-21T16:34:55Z",
"timestamp": 1740155695061,
"version": "3.37.3"
},
"is-referenced-by-count": 1,
"issue": "4",
"issued": {
"date-parts": [
[
2023,
4,
21
]
]
},
"journal-issue": {
"issue": "4",
"published-online": {
"date-parts": [
[
2023,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
4,
21
]
],
"date-time": "2023-04-21T00:00:00Z",
"timestamp": 1682035200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1424-8247/16/4/631/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "631",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
4,
21
]
]
},
"published-online": {
"date-parts": [
[
2023,
4,
21
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1111/joim.13144",
"article-title": "Cytokine storm syndrome in coronavirus disease 2019: A narrative review",
"author": "Gao",
"doi-asserted-by": "crossref",
"first-page": "147",
"journal-title": "J. Intern. Med.",
"key": "ref_1",
"volume": "289",
"year": "2021"
},
{
"DOI": "10.1007/s00296-020-04676-4",
"article-title": "Coronavirus disease 2019 (COVID-19) in autoimmune and inflammatory conditions: Clinical characteristics of poor outcomes",
"author": "Montero",
"doi-asserted-by": "crossref",
"first-page": "1593",
"journal-title": "Rheumatol. Int.",
"key": "ref_2",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.1101/2020.09.14.20194381",
"doi-asserted-by": "crossref",
"key": "ref_3",
"unstructured": "Ramonfaur, D., González-Assad, C.A., and Paredes-Vázquez, J.G. (2020). Pentoxifylline and COVID-19: A Systematic Review. medRxiv."
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "Lancet",
"key": "ref_4",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.3390/jcm10225305",
"doi-asserted-by": "crossref",
"key": "ref_5",
"unstructured": "Feret, W., Nalewajska, M., Wojczyński, Ł., Witkiewicz, W., Kłos, P., Dziedziejko, V., and Pawlik, A. (2021). Pentoxifylline as a Potential Adjuvant Therapy for COVID-19: Impeding the Burden of the Cytokine Storm. J. Clin. Med., 10."
},
{
"DOI": "10.3390/jcm11112951",
"doi-asserted-by": "crossref",
"key": "ref_6",
"unstructured": "Salton, F., Confalonieri, P., Campisciano, G., Cifaldi, R., Rizzardi, C., Generali, D., Pozzan, R., Tavano, S., Bozzi, C., and Lapadula, G. (2022). Cytokine Profiles as Potential Prognostic and Therapeutic Markers in SARS-CoV-2-Induced ARDS. J. Clin. Med., 11."
},
{
"DOI": "10.1016/j.jiph.2021.10.024",
"article-title": "Efficacy of the early treatment with tocilizumab-hydroxychloroquine and tocilizumab-remdesivir in severe COVID-19 Patients",
"author": "Sarhan",
"doi-asserted-by": "crossref",
"first-page": "116",
"journal-title": "J. Infect. Public Health",
"key": "ref_7",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.3390/ijms21155338",
"doi-asserted-by": "crossref",
"key": "ref_8",
"unstructured": "Giorgi, M., Cardarelli, S., Ragusa, F., Saliola, M., Biagioni, S., Poiana, G., Naro, F., and Massimi, M. (2020). Phosphodiesterase Inhibitors: Could They Be Beneficial for the Treatment of COVID-19?. Int. J. Mol. Sci., 21."
},
{
"DOI": "10.3390/medicina58020144",
"doi-asserted-by": "crossref",
"key": "ref_9",
"unstructured": "Zanza, C., Romenskaya, T., Manetti, A.C., Franceschi, F., La Russa, R., Bertozzi, G., Maiese, A., Savioli, G., Volonnino, G., and Longhitano, Y. (2022). Cytokine Storm in COVID-19: Immunopathogenesis and Therapy. Medicina, 58."
},
{
"DOI": "10.3390/jcm11102945",
"doi-asserted-by": "crossref",
"key": "ref_10",
"unstructured": "Marc, F., Moldovan, C.M., Hoza, A., Magheru, S., Ciavoi, G., Farcas, D.M., Sachelarie, L., Calin, G., Romila, L., and Damir, D. (2022). Comparative Study of Cytokine Storm Treatment in Patients with COVID-19 Pneumonia Using Im-munomodulators. J. Clin. Med., 11."
},
{
"DOI": "10.1016/j.mehy.2020.110051",
"article-title": "Harnessing adenosine A2A receptors as a strategy for suppressing the lung inflammation and thrombotic complications of COVID-19: Potential of pentoxifylline and dipyridamole",
"author": "DiNicolantonio",
"doi-asserted-by": "crossref",
"first-page": "110051",
"journal-title": "Med. Hypotheses",
"key": "ref_11",
"volume": "143",
"year": "2020"
},
{
"DOI": "10.3389/fphar.2018.01048",
"article-title": "Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "1048",
"journal-title": "Front. Pharmacol.",
"key": "ref_12",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1128/JVI.72.6.4712-4720.1998",
"article-title": "Inhibition of Phosphodiesterase Type IV Suppresses Human Immunodeficiency Virus Type 1 Replication and Cytokine Production in Primary T Cells: Involvement of NF-κB and NFAT",
"author": "Navarro",
"doi-asserted-by": "crossref",
"first-page": "4712",
"journal-title": "J. Virol.",
"key": "ref_13",
"volume": "72",
"year": "1998"
},
{
"DOI": "10.1097/HJH.0000000000001086",
"article-title": "Effects of pentoxifylline on inflammatory markers and blood pressure: A systematic review and meta-analysis of randomized controlled trials",
"author": "Brie",
"doi-asserted-by": "crossref",
"first-page": "2318",
"journal-title": "J. Hypertens.",
"key": "ref_14",
"volume": "34",
"year": "2016"
},
{
"DOI": "10.1164/ajrccm.159.2.9804085",
"article-title": "Pentoxifylline inhibits TNF-α production from human alveolar macrophages",
"author": "Marques",
"doi-asserted-by": "crossref",
"first-page": "508",
"journal-title": "Am. J. Respir. Crit. Care Med.",
"key": "ref_15",
"volume": "159",
"year": "1999"
},
{
"article-title": "Pentoxifylline and severe acute respiratory syndrome (SARS): A drug to be considered",
"author": "Martin",
"first-page": "SR29",
"journal-title": "Med. Sci. Monit.",
"key": "ref_16",
"volume": "9",
"year": "2003"
},
{
"DOI": "10.1007/s12325-017-0547-2",
"article-title": "Repurposing pentoxifylline for the treatment of fibrosis: An overview",
"author": "Wen",
"doi-asserted-by": "crossref",
"first-page": "1245",
"journal-title": "Adv. Ther.",
"key": "ref_17",
"volume": "34",
"year": "2017"
},
{
"DOI": "10.1100/tsw.2011.22",
"article-title": "Anti-inflammatory and immunosuppressive effects of the A2A adenosine receptor",
"author": "Milne",
"doi-asserted-by": "crossref",
"first-page": "320",
"journal-title": "Sci. World J.",
"key": "ref_18",
"volume": "11",
"year": "2011"
},
{
"DOI": "10.1038/s41577-020-0311-8",
"article-title": "The trinity of COVID-19: Immunity, inflammation and intervention",
"author": "Tay",
"doi-asserted-by": "crossref",
"first-page": "363",
"journal-title": "Nat. Rev. Immunol.",
"key": "ref_19",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1111/dth.13733",
"article-title": "Treatment of COVID-19 with pentoxifylline: Could it be a potential adjuvant therapy?",
"author": "Seirafianpour",
"doi-asserted-by": "crossref",
"first-page": "e13733",
"journal-title": "Dermatol. Ther.",
"key": "ref_20",
"volume": "33",
"year": "2020"
},
{
"DOI": "10.1093/bjaceaccp/mki013",
"article-title": "Acute lung injury: Options to improve oxygenation",
"author": "Mahajan",
"doi-asserted-by": "crossref",
"first-page": "52",
"journal-title": "Contin. Educ. Anaesth. Crit. Care Pain",
"key": "ref_21",
"volume": "5",
"year": "2005"
},
{
"DOI": "10.1016/S0022-4804(03)00219-1",
"article-title": "Pentoxifylline reduces acute lung injury in chronic endotoxemia",
"author": "Michetti",
"doi-asserted-by": "crossref",
"first-page": "92",
"journal-title": "J. Surg. Res.",
"key": "ref_22",
"volume": "115",
"year": "2003"
},
{
"DOI": "10.1111/1755-5922.12076",
"article-title": "Pentoxifylline in heart failure: A meta-analysis of clinical trials",
"author": "Champion",
"doi-asserted-by": "crossref",
"first-page": "159",
"journal-title": "Cardiovasc. Ther.",
"key": "ref_23",
"volume": "32",
"year": "2014"
},
{
"DOI": "10.1111/apt.12279",
"article-title": "Systematic review: Pentoxifylline for the treatment of severe alcoholic hepatitis",
"author": "Parker",
"doi-asserted-by": "crossref",
"first-page": "845",
"journal-title": "Aliment. Pharmacol. Ther.",
"key": "ref_24",
"volume": "37",
"year": "2013"
},
{
"DOI": "10.3892/etm.2014.2132",
"article-title": "Preventive effect of pentoxifylline on contrast-induced acute kidney injury in hypercholesterolemic rats",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "384",
"journal-title": "Exp. Ther. Med.",
"key": "ref_25",
"volume": "9",
"year": "2015"
},
{
"article-title": "Pentoxifylline exerts anti-inflammatory effects on cerebral ischemia reperfusion-induced injury in a rat model via the p38 mitogen-activated protein kinase signaling pathway",
"author": "Dong",
"first-page": "1141",
"journal-title": "Mol. Med. Rep.",
"key": "ref_26",
"volume": "17",
"year": "2018"
},
{
"DOI": "10.1001/archsurg.133.1.94",
"article-title": "Effect of pentoxifylline in severe sepsis: Results of a randomized, double-blind, placebo-controlled study",
"author": "Staubach",
"doi-asserted-by": "crossref",
"first-page": "94",
"journal-title": "Arch. Surg.",
"key": "ref_27",
"volume": "133",
"year": "1998"
},
{
"DOI": "10.1002/14651858.CD004205.pub3",
"doi-asserted-by": "crossref",
"key": "ref_28",
"unstructured": "Pammi, M., and Haque, K.N. (2015). Pentoxifylline for treatment of sepsis and necrotizing enterocolitis in neonates. Cochrane Database Syst. Rev."
},
{
"DOI": "10.22207/JPAM.14.SPL1.23",
"article-title": "Pentoxifylline: An immunomodulatory drug for the treatment of COVID-19",
"author": "Dhameliya",
"doi-asserted-by": "crossref",
"first-page": "861",
"journal-title": "J. Pure Appl. Microbiol.",
"key": "ref_29",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1016/j.mehy.2020.109988",
"article-title": "Repositioning of pentoxifylline as an immunomodulator and regulator of the renin-angiotensin system in the treatment of COVID-19",
"author": "Maldonado",
"doi-asserted-by": "crossref",
"first-page": "109988",
"journal-title": "Med. Hypotheses",
"key": "ref_30",
"volume": "144",
"year": "2020"
},
{
"DOI": "10.1016/j.intimp.2020.107209",
"article-title": "Pentoxifylline decreases serum LDH levels and increases lymphocyte count in COVID-19 patients: Results from an external pilot study",
"author": "Maldonado",
"doi-asserted-by": "crossref",
"first-page": "107209",
"journal-title": "Int. Immunopharmacol.",
"key": "ref_31",
"volume": "90",
"year": "2021"
},
{
"DOI": "10.1159/000512234",
"article-title": "Pentoxifylline: A drug with antiviral and anti-inflammatory effects to be considered in the treatment of coronavirus disease 2019",
"author": "Pashapour",
"doi-asserted-by": "crossref",
"first-page": "98",
"journal-title": "Med. Princ. Pract.",
"key": "ref_32",
"volume": "30",
"year": "2021"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106024",
"article-title": "Extra-respiratory manifestations of COVID-19",
"author": "Lai",
"doi-asserted-by": "crossref",
"first-page": "106024",
"journal-title": "Int. J. Antimicrob. Agents",
"key": "ref_33",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1038/s41572-019-0069-0",
"article-title": "Acute respiratory distress syndrome",
"author": "Matthay",
"doi-asserted-by": "crossref",
"first-page": "18",
"journal-title": "Nat. Rev. Dis. Prim.",
"key": "ref_34",
"volume": "5",
"year": "2019"
},
{
"key": "ref_35",
"unstructured": "Monji, F., Siddiquee, A., and Hashemian, F. (2022, October 20). Potential Role of Methylxanthines as an Adjuvant to COVID-19 Treatment: A Review of Pentoxifylline and Caffeine as the Case of Any Port in the Storm. Authorea Preprints. Available online: https://www.authorea.com/doi/full/10.22541/au.159015270.02586731."
},
{
"DOI": "10.1097/BOR.0000000000000822",
"article-title": "Is severe COVID-19 a cytokine storm syndrome: A hyperinflammatory debate",
"author": "Mehta",
"doi-asserted-by": "crossref",
"first-page": "419",
"journal-title": "Curr. Opin. Rheumatol.",
"key": "ref_36",
"volume": "33",
"year": "2021"
},
{
"DOI": "10.1016/j.cyto.2021.155428",
"article-title": "Interleukin-6, procalcitonin and neutrophil-to-lymphocyte ratio: Potential immune-inflammatory parameters to identify severe and fatal forms of COVID-19",
"author": "Sayah",
"doi-asserted-by": "crossref",
"first-page": "155428",
"journal-title": "Cytokine",
"key": "ref_37",
"volume": "141",
"year": "2021"
},
{
"DOI": "10.1093/eurheartj/ehaa1103",
"article-title": "C-reactive protein and clinical outcomes in patients with COVID-19",
"author": "Smilowitz",
"doi-asserted-by": "crossref",
"first-page": "2270",
"journal-title": "Eur. Heart J.",
"key": "ref_38",
"volume": "42",
"year": "2021"
},
{
"article-title": "Potential usefulness of pentoxifylline, a non-specific phosphodiesterase inhibitor with anti-inflammatory, anti-thrombotic, antioxidant, and anti-fibrogenic properties, in the treatment of SARS-CoV-2",
"author": "Sandoval",
"first-page": "7612",
"journal-title": "Eur. Rev. Med. Pharmacol. Sci.",
"key": "ref_39",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1016/j.csbj.2021.02.009",
"article-title": "Antioxidants and pentoxifylline as coadjuvant measures to standard therapy to improve prognosis of patients with pneumonia by COVID-19",
"author": "Cherit",
"doi-asserted-by": "crossref",
"first-page": "1379",
"journal-title": "Comput. Struct. Biotechnol. J.",
"key": "ref_40",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1016/j.intimp.2021.108227",
"article-title": "Pentoxifylline effects on hospitalized patients with COVID19: A randomized, double-blind clinical trial",
"author": "Azizi",
"doi-asserted-by": "crossref",
"first-page": "108227",
"journal-title": "Int. Immunopharmacol.",
"key": "ref_41",
"volume": "101",
"year": "2021"
},
{
"DOI": "10.1186/s13054-020-03374-8",
"article-title": "Predictive values of neutrophil-to-lymphocyte ratio on disease severity and mortality in COVID-19 patients: A systematic review and meta-analysis",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "647",
"journal-title": "Crit. Care",
"key": "ref_42",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1080/22221751.2020.1746199",
"article-title": "Hypothesis for potential pathogenesis of SARS-CoV-2 infection—A review of immune changes in patients with viral pneumonia",
"author": "Sandoval",
"doi-asserted-by": "crossref",
"first-page": "727",
"journal-title": "Emerg. Microbes Infect.",
"key": "ref_43",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.11613/BM.2021.030501",
"doi-asserted-by": "crossref",
"key": "ref_44",
"unstructured": "Palladino, M. (2021). Complete blood count alterations in COVID-19 patients: A narrative review. Biochem. Med., 31."
},
{
"DOI": "10.1007/s42399-020-00380-3",
"article-title": "COVID-19 infection and haematological involvement: A review of epidemiology, pathophysiology and prognosis of full blood count findings",
"author": "Violetis",
"doi-asserted-by": "crossref",
"first-page": "1089",
"journal-title": "SN Compr. Clin. Med.",
"key": "ref_45",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1186/s43168-020-00045-8",
"article-title": "Leukocyte count in COVID-19: An important consideration",
"author": "Selim",
"doi-asserted-by": "crossref",
"first-page": "1089",
"journal-title": "Egypt. J. Bronchol.",
"key": "ref_46",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1186/s40560-020-00466-z",
"article-title": "D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: A case control study",
"author": "Yao",
"doi-asserted-by": "crossref",
"first-page": "49",
"journal-title": "J. Intensive Care",
"key": "ref_47",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1007/s00281-017-0639-8",
"article-title": "Cytokine storm and sepsis disease pathogenesis",
"author": "Chousterman",
"doi-asserted-by": "crossref",
"first-page": "517",
"journal-title": "Seminars in Immunopathology",
"key": "ref_48",
"volume": "Volume 39",
"year": "2017"
},
{
"article-title": "Current concepts in the diagnosis and management of cytokine release syndrome",
"author": "Lee",
"first-page": "188",
"journal-title": "Blood J. Am. Soc. Hematol.",
"key": "ref_49",
"volume": "124",
"year": "2014"
},
{
"DOI": "10.11613/BM.2021.010502",
"article-title": "Sample size, power and effect size revisited: Simplified and practical approaches in pre-clinical, clinical and laboratory studies",
"author": "Serdar",
"doi-asserted-by": "crossref",
"first-page": "27",
"journal-title": "Biochem. Med.",
"key": "ref_50",
"volume": "31",
"year": "2021"
},
{
"DOI": "10.1016/j.jclinepi.2015.04.014",
"article-title": "A simple formula for the calculation of sample size in pilot studies",
"author": "Viechtbauer",
"doi-asserted-by": "crossref",
"first-page": "1375",
"journal-title": "J. Clin. Epidemiol.",
"key": "ref_51",
"volume": "68",
"year": "2015"
}
],
"reference-count": 51,
"references-count": 51,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1424-8247/16/4/631"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Pentoxifylline Effects on Hospitalized COVID-19 Patients with Cytokine Storm Syndrome: A Randomized Clinical Trial",
"type": "journal-article",
"volume": "16"
}