Patient-reported improvements from use of IMC-2 alone and IMC-2 and Paxlovid® in a Long COVID cohort: a case series
et al., Frontiers in Immunology, doi:10.3389/fimmu.2025.1698271, Jan 2026
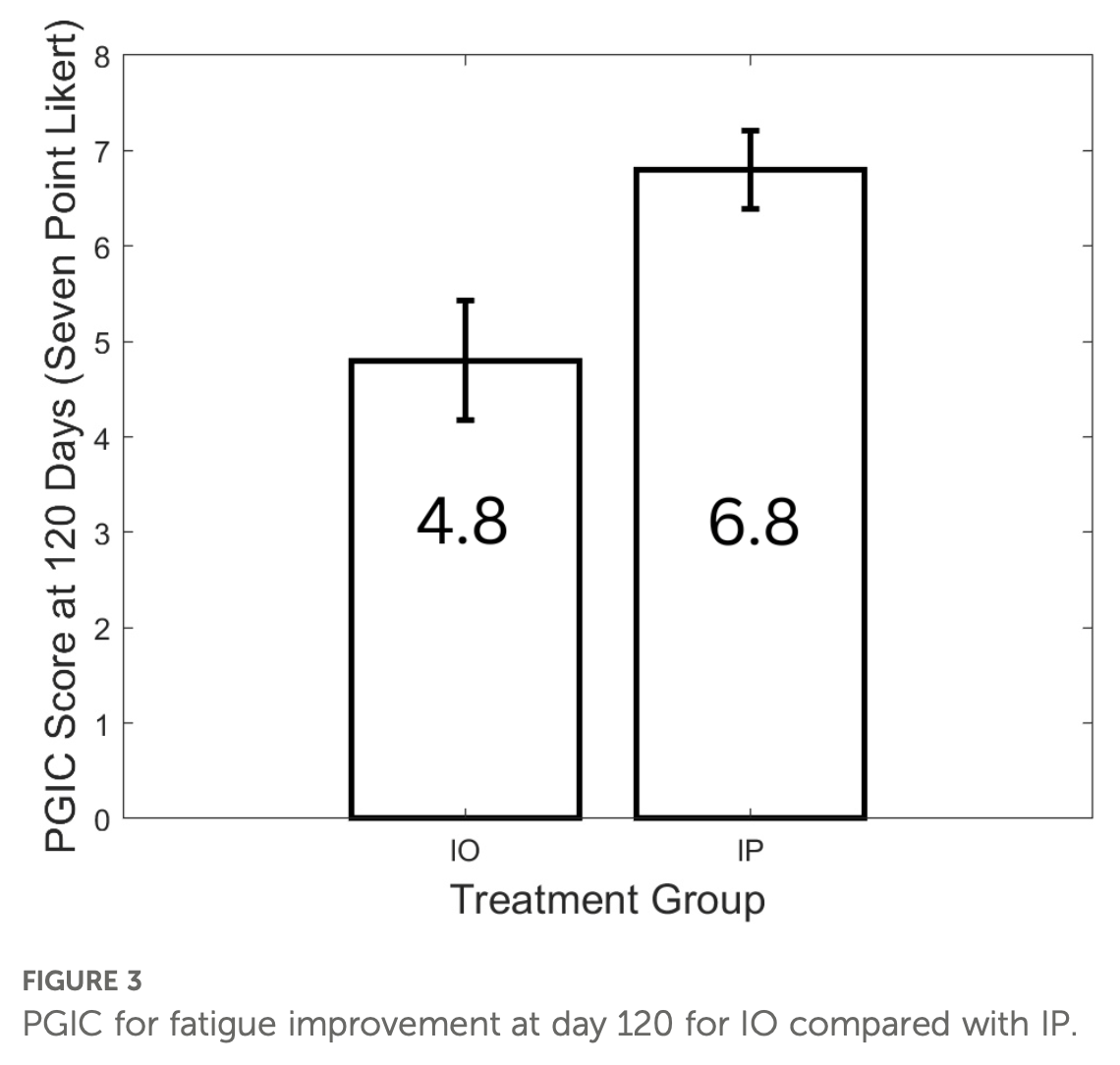
Case series of 24 long COVID outpatients showing greater symptom improvement with a combination of IMC-2 (valacyclovir + celecoxib) plus 15-day paxlovid compared to IMC-2 alone over 120 days.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
Pridgen et al., 5 Jan 2026, retrospective, USA, peer-reviewed, 2 authors, study period April 2022 - February 2024.
Contact: tsasurgery@gmail.com, david.putrino@mountsinai.org.
Patient-reported improvements from use of IMC-2 alone and IMC-2 and Paxlovid® in a Long COVID cohort: a case series
Frontiers in Immunology, doi:10.3389/fimmu.2025.1698271
Introduction: Long COVID (LC) is an infection-associated chronic condition and illness (IACCI) with no currently approved treatments. In order to address SARS-CoV-2 persistence and herpesvirus reactivation, which have been implicated as drivers of LC, sustained use of antiviral combinations may be useful in treating patients with the illness. Methods: A convenience sample of patients undergoing an extended course of antiviral therapy was studied. Patients received either 120 days of IMC-2 only (IO) or 120 days of IMC-2 with the addition of 15 days of Paxlovid (IP), prescribed offlabel at an outpatient clinic for people with LC. The Patient Global Impression of Change (PGIC) was used to measure therapy response over time, with primary focus on fatigue and secondary focus on brain fog and dysautonomia. Visual analog scales (VAS) were also used to track perceived symptom improvements. Results: A total of 27 people with LC were approached for treatment, of whom 24 completed one or both protocols. Twelve received the IO protocol, and 12 received the continuous IP combination. Both groups reported reductions in fatigue on the PGIC, but participants receiving IP experienced a statistically significant improvement compared with those receiving IO (p < 0.0001). Similarly, using a VAS, patients in the IP group reported an average 55.3% (p < 0.0001) greater reduction in fatigue than the IO group. Participants who completed the IP intervention demonstrated durable clinical benefit, with symptom improvements remaining consistent at 120-, 305-, and 731-day follow-ups. Discussion: This small, open-label case series provides pilot evidence supporting the need for a larger trial of combination antivirals for people living with LC. Based on these results, a larger, controlled trial of IMC-2 paired with Paxlovid is recommended.
Ethics statement The studies involving humans were approved by IRCM Office for Human Research Protection of HHS. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Conflict of interest WP is a partner and founder at PridCor Therapeutics, a company that holds a patent covering components that make up IMC-2. The remaining author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement The author(s) declared that generative AI was not used in the creation of this manuscript. Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this..
References
Alfajaro, Choi, Kim, Seo, Kim et al., Activation of COX-2/ PGE2 promotes sapovirus replication via the inhibition of nitric oxide production, J Virol
Cutler, The costs of long COVID, JAMA Health Forum
Davis, Mccorkell, Vogel, Topol, Long COVID: major findings, mechanisms and recommendations, Nat Rev Microbiol, doi:10.1038/s41579-022-00846-2
Deng, Stjohn, Osswald, Brien, Banach et al., Coronaviruses resistant to a 3C-like protease inhibitor are attenuated for replication and pathogenesis, revealing a low genetic barrier but high fitness cost of resistance, Virol, doi:10.1128/JVI.01528-14
Gebhardt, Varnell, Kaufman, Inhibition of cyclooxygenase 2 synthesis suppresses Herpes simplex virus type 1 reactivation, J Ocul Pharmacol Ther, doi:10.1089/jop.2005.21.114
Geisser, Clauw, Strand, Gendreau, Palmer et al., Contributions of change in clinical status parameters to Patient Global Impression of Change (PGIC) scores among persons with fibromyalgia treated with milnacipran, PAIN ®, doi:10.1016/j.pain.2010.02.043
Geng, Bonilla, Hedlin, Jacobson, Tian et al., Nirmatrelvir-ritonavir and symptoms in adults with postacute sequelae of SARS-CoV-2 infection: the STOP-PASC randomized clinical trial, JAMA Internal Med, doi:10.1001/jamainternmed.2024.2007
Ghandi, Gaur, Khera, Kaul, Robertson, COX-2 induces lytic reactivation of EBV through PGE2 by modulating the EP receptor signaling pathway, Virology
Ghaznavi, Mohammadghasemipour, Shirvaliloo, Momeni, Metanat et al., Short-term celecoxib (celebrex) adjuvant therapy: a clinical trial study on COVID-19 patients, Inflammopharmacology, doi:10.1007/s10787-022-01029-4
Hastie, Lowe, Mcauley, Mills, Winter et al., True Prevalence of long COVID in a nationwide, population cohort study, Nat Commun, doi:10.1038/s41467-023-43661-w
Higaki, Watanabe, Itahashi, Shimomura, Cyclooxygenase (COX)inhibiting drug reduces HSV-1 reactivation in the mouse eye model, Curr Eye Res, doi:10.1080/02713680802650377
Hurst, Bolton, Assessing the clinical significance of change scores recorded on subjective outcome measures, J manipulative Physiol Ther, doi:10.1016/j.jmpt.2003.11.003
Inglot, Comparison of the antiviral activity in vitro of some non-steroidal anti-inflammatory drugs, J Gen Virol, doi:10.1099/0022-1317-4-2-203
Iwasaki, Putrino, Why we need a deeper understanding of the pathophysiology of long COVID, Lancet Infect Diseases
Klein, Wood, Jaycox, Dhodapkar, Lu et al., Distinguishing features of long COVID identified through immune profiling, Nature, doi:10.1038/s41586-023-06651-y
Naderi, Naderi, Jamal, Past, Esmaeili et al., The reactivation of the various types of viruses following COVID-19 infection: a systematic review, Future Virol, doi:10.1080/17460794.2025.2483122
Pan, Peto, Restrepo, Preziosi, Sathiyamoorthy et al., Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses, Lancet, doi:10.1016/S0140-6736(22)00519-0
Peluso, Deeks, Mechanisms of long COVID and the path toward therapeutics, Cell, doi:10.1016/j.cell.2024.07.054
Peluso, Ryder, Flavell, Wang, Levi et al., Multimodal molecular imaging reveals tissue-based T cell activation and viral RNA persistence for up to 2 years following COVID-19, Medrxiv, doi:10.1101/2023.07.27.23293177
Proal, Aleman, Bomsel, Brodin, Buggert et al., Targeting the SARS-CoV-2 reservoir in long COVID, Lancet Infect Dis, doi:10.1016/S1473-3099(24)00769-2
Sawano, Bhattacharjee, Caraballo, Khera, Li et al., Nirmatrelvir-ritonavir versus placebo-ritonavir in individuals with long COVID in the USA (PAX LC): a double-blind, randomised, placebo-controlled, phase 2, decentralised trial, Lancet Infect Dis, doi:10.1016/S1473-3099(25)00073-8
Van Der Felz-Cornelis, Turk, Sweetman, Khunti, Gabbay et al., Prevalence of mental health conditions and brain fog in people with long COVID: A systematic review and meta-analysis, Gen Hosp Psychiatry, doi:10.1016/j.genhosppsych.2024.02.009
Yehong, Li, Wang, The role of cyclooxygenase in multiplication and reactivation of HSV-1 in vestibular ganglion cells, ScientificWorldJournal
DOI record:
{
"DOI": "10.3389/fimmu.2025.1698271",
"ISSN": [
"1664-3224"
],
"URL": "http://dx.doi.org/10.3389/fimmu.2025.1698271",
"abstract": "<jats:sec>\n <jats:title>Introduction</jats:title>\n <jats:p>Long COVID (LC) is an infection-associated chronic condition and illness (IACCI) with no currently approved treatments. In order to address SARS-CoV-2 persistence and herpesvirus reactivation, which have been implicated as drivers of LC, sustained use of antiviral combinations may be useful in treating patients with the illness.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>A convenience sample of patients undergoing an extended course of antiviral therapy was studied. Patients received either 120 days of IMC-2 only (IO) or 120 days of IMC-2 with the addition of 15 days of Paxlovid (IP), prescribed off-label at an outpatient clinic for people with LC. The Patient Global Impression of Change (PGIC) was used to measure therapy response over time, with primary focus on fatigue and secondary focus on brain fog and dysautonomia. Visual analog scales (VAS) were also used to track perceived symptom improvements.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>\n A total of 27 people with LC were approached for treatment, of whom 24 completed one or both protocols. Twelve received the IO protocol, and 12 received the continuous IP combination. Both groups reported reductions in fatigue on the PGIC, but participants receiving IP experienced a statistically significant improvement compared with those receiving IO (\n <jats:italic>p</jats:italic>\n &lt; 0.0001). Similarly, using a VAS, patients in the IP group reported an average 55.3% (\n <jats:italic>p</jats:italic>\n &lt; 0.0001) greater reduction in fatigue than the IO group. Participants who completed the IP intervention demonstrated durable clinical benefit, with symptom improvements remaining consistent at 120-, 305-, and 731-day follow-ups.\n </jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Discussion</jats:title>\n <jats:p>This small, open-label case series provides pilot evidence supporting the need for a larger trial of combination antivirals for people living with LC. Based on these results, a larger, controlled trial of IMC-2 paired with Paxlovid is recommended.</jats:p>\n </jats:sec>",
"alternative-id": [
"10.3389/fimmu.2025.1698271"
],
"article-number": "1698271",
"author": [
{
"affiliation": [],
"family": "Pridgen",
"given": "William L.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Putrino",
"given": "David",
"sequence": "additional"
}
],
"container-title": "Frontiers in Immunology",
"container-title-short": "Front. Immunol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2026,
1,
5
]
],
"date-time": "2026-01-05T05:29:55Z",
"timestamp": 1767590995000
},
"deposited": {
"date-parts": [
[
2026,
1,
5
]
],
"date-time": "2026-01-05T05:29:56Z",
"timestamp": 1767590996000
},
"indexed": {
"date-parts": [
[
2026,
1,
5
]
],
"date-time": "2026-01-05T05:34:45Z",
"timestamp": 1767591285910,
"version": "3.48.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2026,
1,
5
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2026,
1,
5
]
],
"date-time": "2026-01-05T00:00:00Z",
"timestamp": 1767571200000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fimmu.2025.1698271/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2026,
1,
5
]
]
},
"published-online": {
"date-parts": [
[
2026,
1,
5
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1038/s41467-023-43661-w",
"article-title": "True Prevalence of long COVID in a nationwide, population cohort study",
"author": "Hastie",
"doi-asserted-by": "publisher",
"first-page": "7892",
"journal-title": "Nat Commun",
"key": "B1",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1016/j.genhosppsych.2024.02.009",
"article-title": "Prevalence of mental health conditions and brain fog in people with long COVID: A systematic review and meta-analysis",
"author": "van der Felz-Cornelis",
"doi-asserted-by": "publisher",
"first-page": "10",
"journal-title": "Gen Hosp Psychiatry",
"key": "B2",
"volume": "88",
"year": "2024"
},
{
"article-title": "The costs of long COVID",
"author": "Cutler",
"first-page": "e221809",
"key": "B3",
"volume-title": "JAMA Health Forum",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(23)00053-1",
"article-title": "Why we need a deeper understanding of the pathophysiology of long COVID",
"author": "Iwasaki",
"doi-asserted-by": "crossref",
"journal-title": "Lancet Infect Diseases",
"key": "B4",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1038/s41586-023-06651-y",
"article-title": "Distinguishing features of long COVID identified through immune profiling",
"author": "Klein",
"doi-asserted-by": "publisher",
"journal-title": "Nature",
"key": "B5",
"volume": "623",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(24)00769-2",
"article-title": "Targeting the SARS-CoV-2 reservoir in long COVID",
"author": "Proal",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Infect Dis",
"key": "B6",
"volume": "25",
"year": "2025"
},
{
"DOI": "10.1001/jamainternmed.2024.2007",
"article-title": "Nirmatrelvir-ritonavir and symptoms in adults with postacute sequelae of SARS-CoV-2 infection: the STOP-PASC randomized clinical trial",
"author": "Geng",
"doi-asserted-by": "publisher",
"journal-title": "JAMA Internal Med",
"key": "B7",
"volume": "184",
"year": "2024"
},
{
"DOI": "10.1016/S1473-3099(25)00073-8",
"article-title": "Nirmatrelvir–ritonavir versus placebo–ritonavir in individuals with long COVID in the USA (PAX LC): a double-blind, randomised, placebo-controlled, phase 2, decentralised trial",
"author": "Sawano",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Infect Dis",
"key": "B8",
"year": "2025"
},
{
"DOI": "10.1016/S0140-6736(22)00519-0",
"article-title": "Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses",
"author": "Pan",
"doi-asserted-by": "publisher",
"journal-title": "Lancet",
"key": "B9",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2024.07.054",
"article-title": "Mechanisms of long COVID and the path toward therapeutics",
"author": "Peluso",
"doi-asserted-by": "publisher",
"journal-title": "Cell",
"key": "B10",
"volume": "187",
"year": "2024"
},
{
"DOI": "10.1080/17460794.2025.2483122",
"article-title": "The reactivation of the various types of viruses following COVID-19 infection: a systematic review",
"author": "Naderi",
"doi-asserted-by": "publisher",
"first-page": "99",
"journal-title": "Future Virol",
"key": "B11",
"volume": "20",
"year": "2025"
},
{
"article-title": "COX-2 induces lytic reactivation of EBV through PGE2 by modulating the EP receptor signaling pathway",
"author": "Ghandi",
"first-page": "006",
"journal-title": "Virology",
"key": "B12",
"volume": "5",
"year": "2015"
},
{
"article-title": "The role of cyclooxygenase in multiplication and reactivation of HSV-1 in vestibular ganglion cells",
"author": "Yehong",
"first-page": "912640",
"journal-title": "ScientificWorldJournal",
"key": "B13",
"volume": "2014",
"year": "2014"
},
{
"DOI": "10.1128/JVI.01656-16",
"article-title": "Activation of COX-2/PGE2 promotes sapovirus replication via the inhibition of nitric oxide production",
"author": "Alfajaro",
"doi-asserted-by": "crossref",
"journal-title": "J Virol",
"key": "B14",
"volume": "91",
"year": "2017"
},
{
"DOI": "10.1099/0022-1317-4-2-203",
"article-title": "Comparison of the antiviral activity in vitro of some non-steroidal anti-inflammatory drugs",
"author": "Inglot",
"doi-asserted-by": "publisher",
"journal-title": "J Gen Virol",
"key": "B15",
"volume": "4",
"year": "1969"
},
{
"DOI": "10.1080/02713680802650377",
"article-title": "Cyclooxygenase (COX)-inhibiting drug reduces HSV-1 reactivation in the mouse eye model",
"author": "Higaki",
"doi-asserted-by": "publisher",
"journal-title": "Curr Eye Res",
"key": "B16",
"volume": "34",
"year": "2009"
},
{
"DOI": "10.1089/jop.2005.21.114",
"article-title": "Inhibition of cyclooxygenase 2 synthesis suppresses Herpes simplex virus type 1 reactivation",
"author": "Gebhardt",
"doi-asserted-by": "publisher",
"journal-title": "J Ocul Pharmacol Ther",
"key": "B17",
"volume": "21",
"year": "2005"
},
{
"DOI": "10.1007/s10787-022-01029-4",
"article-title": "Short-term celecoxib (celebrex) adjuvant therapy: a clinical trial study on COVID-19 patients",
"author": "Ghaznavi",
"doi-asserted-by": "publisher",
"journal-title": "Inflammopharmacology",
"key": "B18",
"volume": "30",
"year": "2022"
},
{
"DOI": "10.1101/2023.07.27.23293177",
"article-title": "Multimodal molecular imaging reveals tissue-based T cell activation and viral RNA persistence for up to 2 years following COVID-19",
"author": "Peluso",
"doi-asserted-by": "publisher",
"journal-title": "Medrxiv",
"key": "B19",
"year": "2023"
},
{
"DOI": "10.1128/JVI.01528-14",
"article-title": "Coronaviruses resistant to a 3C-like protease inhibitor are attenuated for replication and pathogenesis, revealing a low genetic barrier but high fitness cost of resistance",
"author": "Deng",
"doi-asserted-by": "publisher",
"journal-title": "Virol",
"key": "B20",
"volume": "88",
"year": "2014"
},
{
"DOI": "10.1016/j.jmpt.2003.11.003",
"article-title": "Assessing the clinical significance of change scores recorded on subjective outcome measures",
"author": "Hurst",
"doi-asserted-by": "publisher",
"first-page": "26",
"journal-title": "J manipulative Physiol Ther",
"key": "B21",
"volume": "27",
"year": "2004"
},
{
"DOI": "10.1016/j.pain.2010.02.043",
"article-title": "Contributions of change in clinical status parameters to Patient Global Impression of Change (PGIC) scores among persons with fibromyalgia treated with milnacipran",
"author": "Geisser",
"doi-asserted-by": "publisher",
"journal-title": "PAIN®",
"key": "B22",
"volume": "149",
"year": "2010"
},
{
"DOI": "10.1038/s41579-022-00846-2",
"article-title": "Long COVID: major findings, mechanisms and recommendations",
"author": "Davis",
"doi-asserted-by": "publisher",
"journal-title": "Nat Rev Microbiol",
"key": "B23",
"volume": "21",
"year": "2023"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fimmu.2025.1698271/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Patient-reported improvements from use of IMC-2 alone and IMC-2 and Paxlovid® in a Long COVID cohort: a case series",
"type": "journal-article",
"update-policy": "https://doi.org/10.3389/crossmark-policy",
"volume": "16"
}