Nirmatrelvir-Ritonavir and Symptoms in Adults With Postacute Sequelae of SARS-CoV-2 Infection
et al., JAMA Internal Medicine, doi:10.1001/jamainternmed.2024.2007, STOP-PASC, NCT05576662, Jun 2024
RCT 155 adults with long COVID (PASC) showing no significant benefit with paxlovid compared to ritonavir.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
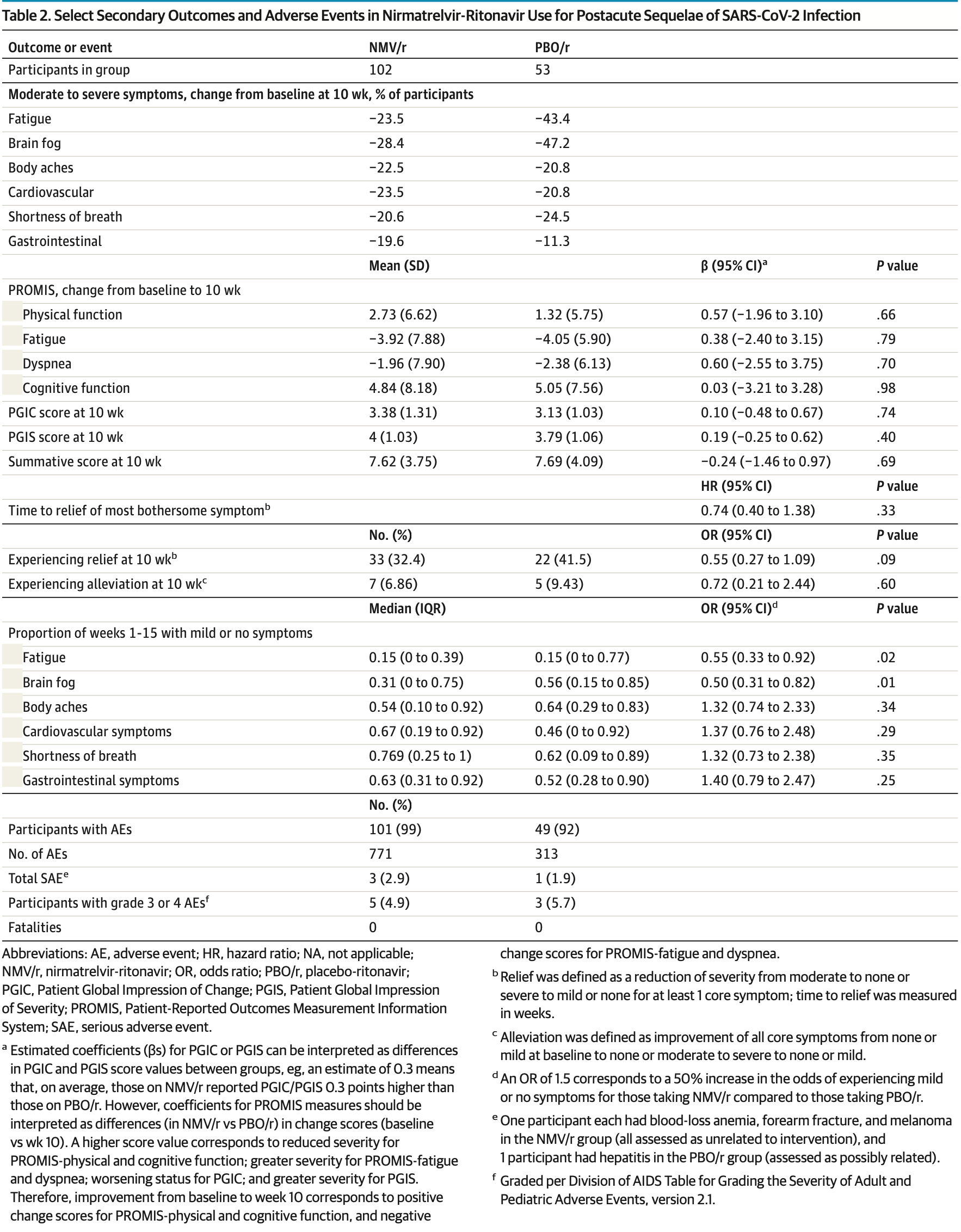
no relief at week 10, 24.3% higher, RR 1.24, p = 0.04, treatment 69 of 102 (67.6%), control 30 of 53 (56.6%), adjusted per study, inverted to make RR<1 favor treatment, odds ratio converted to relative risk.
|
|
no alleviation at week 10, 3.3% higher, RR 1.03, p = 0.62, treatment 95 of 102 (93.1%), control 47 of 53 (88.7%), adjusted per study, inverted to make RR<1 favor treatment, odds ratio converted to relative risk.
|
|
mild/no symp., 3.4% higher, RR 1.03, p = 0.88, treatment 102, control 53, adjusted per study, all symptoms combined.
|
|
mild/no symp., 81.8% higher, OR 1.82, p = 0.02, treatment 102, control 53, adjusted per study, inverted to make OR<1 favor treatment, fatigue, RR approximated with OR.
|
|
mild/no symp., 100% higher, OR 2.00, p = 0.005, treatment 102, control 53, adjusted per study, inverted to make OR<1 favor treatment, brain fog, RR approximated with OR.
|
|
mild/no symp., 24.2% lower, OR 0.76, p = 0.35, treatment 102, control 53, adjusted per study, inverted to make OR<1 favor treatment, body aches, RR approximated with OR.
|
|
mild/no symp., 27.0% lower, OR 0.73, p = 0.30, treatment 102, control 53, adjusted per study, inverted to make OR<1 favor treatment, cardiovascular, RR approximated with OR.
|
|
mild/no symp., 24.2% lower, OR 0.76, p = 0.36, treatment 102, control 53, adjusted per study, inverted to make OR<1 favor treatment, dyspnea, RR approximated with OR.
|
|
mild/no symp., 28.6% lower, OR 0.71, p = 0.25, treatment 102, control 53, adjusted per study, inverted to make OR<1 favor treatment, gastrointestinal, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Geng et al., 7 Jun 2024, Double Blind Randomized Controlled Trial, USA, peer-reviewed, 33 authors, study period 8 November, 2022 - 12 September, 2023, trial NCT05576662 (history) (STOP-PASC).
Contact: geng@stanford.edu, usingh@stanford.edu.
Nirmatrelvir-Ritonavir and Symptoms in Adults With Postacute Sequelae of SARS-CoV-2 Infection
JAMA Internal Medicine, doi:10.1001/jamainternmed.2024.2007
IMPORTANCE There is an urgent need to identify treatments for postacute sequelae of SARS-CoV-2 infection (PASC). OBJECTIVE To assess the efficacy of a 15-day course of nirmatrelvir-ritonavir in reducing the severity of select PASC symptoms.
DESIGN, SETTING, AND PARTICIPANTS This was a 15-week blinded, placebo-controlled, randomized clinical trial conducted from November 2022 to September 2023 at Stanford University (California). The participants were adults with moderate to severe PASC symptoms of 3 months or longer duration. INTERVENTIONS Participants were randomized 2:1 to treatment with oral nirmatrelvirritonavir (NMV/r, 300 mg and 100 mg) or with placebo-ritonavir (PBO/r) twice daily for 15 days.
MAIN OUTCOMES AND MEASURES Primary outcome was a pooled severity of 6 PASC symptoms (fatigue, brain fog, shortness of breath, body aches, gastrointestinal symptoms, and cardiovascular symptoms) based on a Likert scale score at 10 weeks. Secondary outcomes included symptom severity at different time points, symptom burden and relief, patient global measures, Patient-Reported Outcomes Measurement Information System (PROMIS) measures, orthostatic vital signs, and sit-to-stand test change from baseline.
RESULTS Of the 155 participants (median [IQR] age, 43 [34-54] years; 92 [59%] females), 102 were randomized to the NMV/r group and 53 to the PBO/r group. Nearly all participants (n = 153) had received the primary series for COVID-19 vaccination. Mean (SD) time between index SARS-CoV-2 infection and randomization was 17.5 (9.1) months. There was no statistically significant difference in the model-derived severity outcome pooled across the 6 core symptoms at 10 weeks between the NMV/r and PBO/r groups. No statistically significant between-group differences were found at 10 weeks in the Patient Global Impression of Severity or Patient Global Impression of Change scores, summative symptom scores, and change from baseline to 10 weeks in PROMIS fatigue, dyspnea, cognitive function, and physical function measures. Adverse event rates were similar in NMV/r and PBO/r groups and mostly of low grade.
CONCLUSIONS AND RELEVANCE The results of this randomized clinical trial showed that a 15-day course of NMV/r in a population of patients with PASC was generally safe but did not demonstrate a significant benefit for improving select PASC symptoms in a mostly vaccinated cohort with protracted symptom duration. Further studies are needed to determine the role of antivirals in the treatment of PASC.
Author Contributions: Drs Geng and Singh had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs Bonilla, Hedlin, and Jacobson contributed equally. Concept and design: Geng, Bonilla, Hedlin, Jacobson, Jagannathan, Yang, Subramanian, Liang, Desai, Pathak, Banerjee, Lopez, De Jesus, Utz, Singh. Acquisition, analysis, or interpretation of data: Geng, Bonilla, Hedlin, Jacobson, Tian, Jagannathan, Yang, Subramanian, Liang, Shen, Deng, Shaw, Botzheim, Desai, Jazayeri, Thai, O'Donnell, Mohapatra, Leang, Reynolds, Brooks, Bhatt, Shafer, Miglis, Quach, Tiwari, Lopez, Charnas, Singh. Drafting of the manuscript: Geng, Bonilla, Hedlin, Jacobson, Tian, Liang, Shaw, Jazayeri, Thai, Reynolds, Brooks, Bhatt, De Jesus, Charnas, Singh. Critical review of the manuscript for important intellectual content: Geng, Bonilla, Hedlin, Jacobson, Jagannathan, Yang, Subramanian, Liang, Shen, Deng, Shaw, Botzheim, Desai, Pathak, Jazayeri, O'Donnell, Mohapatra, Leang, Shafer, Miglis, Quach, Tiwari, Banerjee, Lopez, De Jesus, Charnas, Utz, Singh.
Conflict of
References
Badran, Gaudin, Struillou, Amador, Soueidan, Periodontal pockets: a potential reservoir for SARS-CoV-2?, Med Hypotheses, doi:10.1016/j.mehy.2020.109907
Ballouz, Menges, Anagnostopoulos, Recovery and symptom trajectories up to two years after SARS-CoV-2 infection: population based, longitudinal cohort study, BMJ, doi:10.1136/bmj-2022-074425
Boglione, Meli, Poletti, Risk factors and incidence of Long-COVID syndrome in hospitalized patients: does remdesivir have a protective effect?, QJM, doi:10.1093/qjmed/hcab297
Bohannon, Crouch, 1-Minute Sit-to-Stand Test: systematic review of procedures, performance, and clinimetric properties, J Cardiopulm Rehabil Prev, doi:10.1097/HCR.0000000000000336
Bonet, Vélez, Jordà, MAR Post-COVID-19 Unit. Treatment of COVID-19 during the acute phase in hospitalized patients decreases post-acute sequelae of COVID-19, J Clin Med, doi:10.3390/jcm12124158
Bonilla, Peluso, Rodgers, Therapeutic trials for long COVID-19: A call to action from the interventions taskforce of the RECOVER initiative, Front Immunol, doi:10.3389/fimmu.2023.1129459
Bonilla, Quach, Tiwari, Myalgic encephalomyelitis/chronic fatigue syndrome is common in post-acute sequelae of SARS-CoV-2 infection (PASC): results from a post-COVID-19 multidisciplinary clinic, Front Neurol, doi:10.3389/fneur.2023.1090747
Brodin, /ritonavir compared with placebo/ritonavir in non-hospitalized adult participants suffering from post-COVID
Byambasuren, Stehlik, Clark, Alcorn, Glasziou, Effect of covid-19 vaccination on long COVID: systematic review, BMJ Med, doi:10.1136/bmjmed-2022-000385
Cevik, Tate, Lloyd, Maraolo, Schafers et al., SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis, Lancet Microbe, doi:10.1016/S2666-5247(20)30172-5
Cheung, Goh, Lim, Residual SARS-CoV-2 viral antigens detected in GI and hepatic tissues from five recovered patients with COVID-19, Gut, doi:10.1136/gutjnl-2021-324280
Choi, Choudhary, Regan, Persistence and evolution of SARS-CoV-2 in an immunocompromised host, N Engl J Med, doi:10.1056/NEJMc2031364
Davis, Assaf, Mccorkell, Characterizing long COVID in an international cohort: 7 months of symptoms and their impact, EClinicalMedicine, doi:10.1016/j.eclinm.2021.101019
Davis, Mccorkell, Vogel, Topol, Long COVID: major findings, mechanisms and recommendations, Nat Rev Microbiol, doi:10.1038/s41579-022-00846-2
Diseases, The Lancet Infectious Diseases. Where are the long COVID trials?, Lancet Infect Dis, doi:10.1016/S1473-3099(23)00440-1
Durstenfeld, Peluso, Lin, Association of nirmatrelvir for acute SARS-CoV-2 infection with subsequent long COVID symptoms in an observational cohort study, J Med Virol, doi:10.1002/jmv.29333
Fontana, Villamagna, Sikka, Mcgregor, Understanding viral shedding of severe acute respiratory coronavirus virus 2 (SARS-CoV-2): review of current literature, Infect Control Hosp Epidemiol, doi:10.1017/ice.2020.1273
Geng, Bonilla, Shafer, Miglis, Yang, The use of nirmatrelvir-ritonavir in a case of breakthrough long COVID, Exploratory Research and Hypothesis in Medicine, doi:10.14218/ERHM.2022.00045
Goh, Lim, Fernaíndez, Case report: persistence of residual antigen and RNA of the SARS-CoV-2 virus in tissues of two patients with long COVID, Front Immunol, doi:10.3389/fimmu.2022.939989
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19
Hansen, Mogensen, Agergaard, High-dose coenzyme Q10 therapy versus placebo in patients with post COVID-19 condition: a randomized, phase 2, crossover trial, Lancet Reg Health Eur, doi:10.1016/j.lanepe.2022.100539
Hanson, Abbafati, Aerts, Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021, JAMA, doi:10.1001/jama.2022.18931?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamainternmed.2024.2007
Healthmeasures, Patient-Reported Outcomes Measurement Information System
Henrich, experiencing long COVID (PREVAIL-LC)
Huang, Yao, Gu, 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study, Lancet, doi:10.1016/S0140-6736(21)01755-4
Hughes, Haroon, Subramanian, Development and validation of the symptom burden questionnaire for long COVID (SBQ-LC): rasch analysis, BMJ, doi:10.1136/bmj-2022-070230
Ioannou, Berry, Rajeevan, Effectiveness of nirmatrelvir-ritonavir against the development of post-COVID-19 conditions among US veterans: a target trial emulation, Ann Intern Med, doi:10.7326/M23-1394
Krumholz, An Interventional decentralized phase 2, randomized, double-blind, 2-arm study to investigate the efficacy and safety of orally administered nirmatrelvir/ritonavir compared with placebo/ritonavir in participants with long COVID
Larsen, Stiles, Shaik, Characterization of autonomic symptom burden in long COVID: a global survey of 2,314 adults, Front Neurol, doi:10.3389/fneur.2022.1012668
Lau, Su, Lau, A symbiotic preparation (SIM01) for post-acute COVID-19 syndrome in Hong Kong (RECOVERY): a randomised, double-blind, placebo-controlled trial, Lancet Infect Dis, doi:10.1016/S1473-3099(23)00685-0
Lopez-Leon, Wegman-Ostrosky, Perelman, More than 50 long-term effects of COVID-19: a systematic review and meta-analysis, Sci Rep, doi:10.1038/s41598-021-95565-8
Malesevic, Sievi, Baumgartner, Impaired health-related quality of life in long-COVID syndrome after mild to moderate COVID-19, Sci Rep, doi:10.1038/s41598-023-34678-8
Mantovani, Morrone, Patrono, COVID-19 Commission of the Accademia Nazionale dei Lincei. Long COVID: where we stand and challenges ahead, Cell Death Differ, doi:10.1038/s41418-022-01052-6
Mohandas, Jagannathan, Henrich, RECOVER Mechanistic Pathways Task Force. Immune mechanisms underlying COVID-19 pathology and post-acute sequelae of SARS-CoV-2 infection (PASC), Elife, doi:10.7554/eLife.86014
Natarajan, Zlitni, Brooks, Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection, Med, doi:10.1016/j.medj.2022.04.001
Nevalainen, Horstia, Laakkonen, Effect of remdesivir post hospitalization for COVID-19 infection from the randomized SOLIDARITY Finland trial, Nat Commun, doi:10.1038/s41467-022-33825-5
O'brien, Brown, Mcduff, Conceptualising the episodic nature of disability among adults living with long COVID: a qualitative study, BMJ Glob Health, doi:10.1136/bmjgh-2022-011276
O'hare, Vig, Iwashyna, Complexity and challenges of the clinical diagnosis and management of long COVID, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.40332?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamainternmed.2024.2007
O'mahoney, Routen, Gillies, The prevalence and long-term health effects of long COVID among hospitalised and non-hospitalised populations: a systematic review and meta-analysis, EClinicalMedicine, doi:10.1016/j.eclinm.2022.101762
Peluso, An exploratory, randomized, double-blind placebo-controlled study to assess the safety of an anti-SARS-CoV-2 monoclonal antibody and response to treatment in individuals with long COVID (OutSMART-LC)
Peluso, Anglin, Durstenfeld, Effect of oral nirmatrelvir on long COVID symptoms: 4 cases and rationale for systematic studies, Pathog Immun, doi:10.20411/pai.v7i1.518
Peluso, Deeks, Mustapic, SARS-CoV-2 and mitochondrial proteins in neural-derived exosomes of COVID-19, Ann Neurol, doi:10.1002/ana.26350
Phillips, Williams, Confronting our next national health disaster: long-haul COVID, N Engl J Med, doi:10.1056/NEJMp2109285
Proal, Van Elzakker, Aleman, SARS-CoV-2 reservoir in post-acute sequelae of COVID-19 (PASC), Nat Immunol, doi:10.1038/s41590-023-01601-2
Proal, Van Elzakker, Long COVID or Post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms, Front Microbiol, doi:10.3389/fmicb.2021.698169
Roden, Boland, Johnson, Late complications of COVID-19, Arch Pathol Lab Med, doi:10.5858/arpa.2021-0519-SA
Schultheiß, Willscher, Paschold, Liquid biomarkers of macrophage dysregulation and circulating spike protein illustrate the biological heterogeneity in patients with post-acute sequelae of COVID-19, J Med Virol, doi:10.1002/jmv.28364
Servier, Porcher, Pane, Ravaud, Tran, Trajectories of the evolution of post-COVID-19 condition, up to two years after symptoms onset, Int J Infect Dis, doi:10.1016/j.ijid.2023.05.007
Stein, Ramelli, Grazioli, NIH COVID-19 Autopsy Consortium. SARS-CoV-2 infection and persistence in the human body and brain at autopsy, Nature, doi:10.1038/s41586-022-05542-y
Su, Yuan, Chen, ISB-Swedish COVID-19 Biobanking Unit. Multiple early factors anticipate post-acute COVID-19 sequelae, Cell, doi:10.1016/j.cell.2022.01.014
Subramanian, Nirantharakumar, Hughes, Symptoms and risk factors for long COVID in non-hospitalized adults, Nat Med, doi:10.1038/s41591-022-01909-w
Swank, Senussi, Manickas-Hill, Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae, Clin Infect Dis, doi:10.1093/cid/ciac722
Thaweethai, Jolley, Karlson, Development of a definition of postacute sequelae of SARS-CoV-2 infection, JAMA, doi:10.1001/jama.2023.8823?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamainternmed.2024.2007
Tran, Porcher, Pane, Ravaud, Course of post COVID-19 disease symptoms over time in the COMPARE long COVID prospective e-cohort, Nat Commun, doi:10.1038/s41467-022-29513-z
Trypsteen, Van Cleemput, Snippenberg, Gerlo, Vandekerckhove, On the whereabouts of SARS-CoV-2 in the human body: a systematic review, PLoS Pathog, doi:10.1371/journal.ppat.1009037
Tsampasian, Elghazaly, Chattopadhyay, Risk factors associated with post-COVID-19 condition: a systematic review and meta-analysis, JAMA Intern Med, doi:10.1001/jamainternmed.2023.0750?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamainternmed.2024.2007
Vibholm, Nielsen, Pahus, SARS-CoV-2 persistence is associated with antigen-specific CD8 T-cell responses, EBioMedicine, doi:10.1016/j.ebiom.2021.103230
Wei, Johnson, Combining dependent tests with incomplete repeated measurements, Biometrika, doi:10.1093/biomet/72.2.359
Xie, Choi, Al-Aly, Association of treatment with nirmatrelvir and the risk of post-COVID-19 condition, JAMA Intern Med, doi:10.1001/jamainternmed.2023.0743?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamainternmed.2024.2007
Xie, Choi, Al-Aly, Molnupiravir and risk of post-acute sequelae of COVID-19: cohort study, BMJ, doi:10.1136/bmj-2022-074572
Xu, Tian, Wei, Combining dependent tests for linkage or association across multiple phenotypic traits, Biostatistics, doi:10.1093/biostatistics/4.2.223
Zimmerman, RECOVER-VITAL: a platform protocol for evaluation of interventions for viral persistence, viral reactivation, and immune dysregulation in post-acute sequelae of SARS-CoV-2 infection (PASC)
Zollner, Koch, Jukic, Postacute COVID-19 is characterized by gut viral antigen persistence in inflammatory bowel diseases, Gastroenterology, doi:10.1053/j.gastro.2022.04.037
DOI record:
{
"DOI": "10.1001/jamainternmed.2024.2007",
"ISSN": [
"2168-6106"
],
"URL": "http://dx.doi.org/10.1001/jamainternmed.2024.2007",
"abstract": "<jats:sec><jats:title>Importance</jats:title><jats:p>There is an urgent need to identify treatments for postacute sequelae of SARS-CoV-2 infection (PASC).</jats:p></jats:sec><jats:sec><jats:title>Objective</jats:title><jats:p>To assess the efficacy of a 15-day course of nirmatrelvir-ritonavir in reducing the severity of select PASC symptoms.</jats:p></jats:sec><jats:sec><jats:title>Design, Setting, and Participants</jats:title><jats:p>This was a 15-week blinded, placebo-controlled, randomized clinical trial conducted from November 2022 to September 2023 at Stanford University (California). The participants were adults with moderate to severe PASC symptoms of 3 months or longer duration.</jats:p></jats:sec><jats:sec><jats:title>Interventions</jats:title><jats:p>Participants were randomized 2:1 to treatment with oral nirmatrelvir-ritonavir (NMV/r, 300 mg and 100 mg) or with placebo-ritonavir (PBO/r) twice daily for 15 days.</jats:p></jats:sec><jats:sec><jats:title>Main Outcomes and Measures</jats:title><jats:p>Primary outcome was a pooled severity of 6 PASC symptoms (fatigue, brain fog, shortness of breath, body aches, gastrointestinal symptoms, and cardiovascular symptoms) based on a Likert scale score at 10 weeks. Secondary outcomes included symptom severity at different time points, symptom burden and relief, patient global measures, Patient-Reported Outcomes Measurement Information System (PROMIS) measures, orthostatic vital signs, and sit-to-stand test change from baseline.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Of the 155 participants (median [IQR] age, 43 [34-54] years; 92 [59%] females), 102 were randomized to the NMV/r group and 53 to the PBO/r group. Nearly all participants (n = 153) had received the primary series for COVID-19 vaccination. Mean (SD) time between index SARS-CoV-2 infection and randomization was 17.5 (9.1) months. There was no statistically significant difference in the model-derived severity outcome pooled across the 6 core symptoms at 10 weeks between the NMV/r and PBO/r groups. No statistically significant between-group differences were found at 10 weeks in the Patient Global Impression of Severity or Patient Global Impression of Change scores, summative symptom scores, and change from baseline to 10 weeks in PROMIS fatigue, dyspnea, cognitive function, and physical function measures. Adverse event rates were similar in NMV/r and PBO/r groups and mostly of low grade.</jats:p></jats:sec><jats:sec><jats:title>Conclusions and Relevance</jats:title><jats:p>The results of this randomized clinical trial showed that a 15-day course of NMV/r in a population of patients with PASC was generally safe but did not demonstrate a significant benefit for improving select PASC symptoms in a mostly vaccinated cohort with protracted symptom duration. Further studies are needed to determine the role of antivirals in the treatment of PASC.</jats:p></jats:sec><jats:sec><jats:title>Trial Registration</jats:title><jats:p>ClinicalTrials.gov Identifier: <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://classic.clinicaltrials.gov/ct2/show/NCT05576662\">NCT05576662</jats:ext-link></jats:p></jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Geng",
"given": "Linda N.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Bonilla",
"given": "Hector",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Hedlin",
"given": "Haley",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
},
{
"name": "Kaiser Permanente Northern California Division of Research, Oakland"
}
],
"family": "Jacobson",
"given": "Karen B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biomedical Data Science, Stanford School of Medicine, Stanford, California"
}
],
"family": "Tian",
"given": "Lu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Jagannathan",
"given": "Prasanna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Yang",
"given": "Phillip C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Subramanian",
"given": "Aruna K.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Liang",
"given": "Jane W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Shen",
"given": "Sa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Deng",
"given": "Yaowei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Shaw",
"given": "Blake J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Botzheim",
"given": "Bren",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Desai",
"given": "Manisha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Pathak",
"given": "Divya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Jazayeri",
"given": "Yasmin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Thai",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
}
],
"family": "O’Donnell",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Mohaptra",
"given": "Sukanya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Leang",
"given": "Zenita",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Reynolds",
"given": "Gabriella Z. M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Brooks",
"given": "Erin F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Bhatt",
"given": "Ami S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Shafer",
"given": "Robert W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Neurology and Neurological Sciences, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Miglis",
"given": "Mitchell G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Stanford University, Stanford, California"
}
],
"family": "Quach",
"given": "Tom",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Stanford University, Stanford, California"
}
],
"family": "Tiwari",
"given": "Anushri",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pfizer Research and Development, Pfizer Inc, Cambridge, Massachusetts"
}
],
"family": "Banerjee",
"given": "Anindita",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Research Collaborations COE, Worldwide Medical and Safety, Pfizer Inc, Groton, Connecticut"
}
],
"family": "Lopez",
"given": "Rene N.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Strategic Planning, Worldwide Medical and Safety, Pfizer Inc, New York, New York"
}
],
"family": "De Jesus",
"given": "Magdia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Research Collaborations COE, Worldwide Medical and Safety, Pfizer Inc, Groton, Connecticut"
}
],
"family": "Charnas",
"given": "Lawrence R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
},
{
"name": "Institute for Immunity, Transplantation and Infection, Stanford University, Stanford, California"
}
],
"family": "Utz",
"given": "Paul J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University School of Medicine, Stanford, California"
},
{
"name": "Department of Microbiology and Immunology, Stanford University School of Medicine, Stanford, California"
}
],
"family": "Singh",
"given": "Upinder",
"sequence": "additional"
}
],
"container-title": "JAMA Internal Medicine",
"container-title-short": "JAMA Intern Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
6,
7
]
],
"date-time": "2024-06-07T19:30:40Z",
"timestamp": 1717788640000
},
"deposited": {
"date-parts": [
[
2024,
6,
7
]
],
"date-time": "2024-06-07T19:30:48Z",
"timestamp": 1717788648000
},
"indexed": {
"date-parts": [
[
2024,
6,
8
]
],
"date-time": "2024-06-08T00:36:05Z",
"timestamp": 1717806965898
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
6,
7
]
]
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jamainternalmedicine/articlepdf/2819901/jamainternal_geng_2024_oi_240036_1717508980.16662.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"prefix": "10.1001",
"published": {
"date-parts": [
[
2024,
6,
7
]
]
},
"published-online": {
"date-parts": [
[
2024,
6,
7
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1001/jama.2022.18931",
"article-title": "Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021.",
"author": "Wulf Hanson",
"doi-asserted-by": "publisher",
"first-page": "1604",
"issue": "16",
"journal-title": "JAMA",
"key": "ioi240036r1",
"volume": "328",
"year": "2022"
},
{
"DOI": "10.1016/j.eclinm.2022.101762",
"article-title": "The prevalence and long-term health effects of long COVID among hospitalised and non-hospitalised populations: a systematic review and meta-analysis.",
"author": "O’Mahoney",
"doi-asserted-by": "publisher",
"journal-title": "EClinicalMedicine",
"key": "ioi240036r2",
"volume": "55",
"year": "2022"
},
{
"DOI": "10.1038/s41598-023-34678-8",
"article-title": "Impaired health-related quality of life in long-COVID syndrome after mild to moderate COVID-19.",
"author": "Malesevic",
"doi-asserted-by": "publisher",
"first-page": "7717",
"issue": "1",
"journal-title": "Sci Rep",
"key": "ioi240036r4",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1016/j.eclinm.2021.101019",
"article-title": "Characterizing long COVID in an international cohort: 7 months of symptoms and their impact.",
"author": "Davis",
"doi-asserted-by": "publisher",
"journal-title": "EClinicalMedicine",
"key": "ioi240036r5",
"volume": "38",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)01755-4",
"article-title": "1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study.",
"author": "Huang",
"doi-asserted-by": "publisher",
"first-page": "747",
"issue": "10302",
"journal-title": "Lancet",
"key": "ioi240036r6",
"volume": "398",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2022.40332",
"article-title": "Complexity and challenges of the clinical diagnosis and management of long COVID.",
"author": "O’Hare",
"doi-asserted-by": "publisher",
"issue": "11",
"journal-title": "JAMA Netw Open",
"key": "ioi240036r7",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1056/NEJMp2109285",
"article-title": "Confronting our next national health disaster: long-haul COVID.",
"author": "Phillips",
"doi-asserted-by": "publisher",
"first-page": "577",
"issue": "7",
"journal-title": "N Engl J Med",
"key": "ioi240036r8",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1038/s41418-022-01052-6",
"article-title": "Long COVID: where we stand and challenges ahead.",
"author": "Mantovani",
"doi-asserted-by": "publisher",
"first-page": "1891",
"issue": "10",
"journal-title": "Cell Death Differ",
"key": "ioi240036r9",
"volume": "29",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(23)00440-1",
"article-title": "Where are the long COVID trials?",
"author": "Diseases",
"doi-asserted-by": "publisher",
"first-page": "879",
"issue": "8",
"journal-title": "Lancet Infect Dis",
"key": "ioi240036r10",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1016/j.lanepe.2022.100539",
"article-title": "High-dose coenzyme Q10 therapy versus placebo in patients with post COVID-19 condition: a randomized, phase 2, crossover trial.",
"author": "Hansen",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Reg Health Eur",
"key": "ioi240036r11",
"volume": "24",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(23)00685-0",
"article-title": "A symbiotic preparation (SIM01) for post-acute COVID-19 syndrome in Hong Kong (RECOVERY): a randomised, double-blind, placebo-controlled trial.",
"author": "Lau",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Infect Dis",
"key": "ioi240036r12",
"year": "2023"
},
{
"DOI": "10.3389/fimmu.2023.1129459",
"article-title": "Therapeutic trials for long COVID-19: A call to action from the interventions taskforce of the RECOVER initiative.",
"author": "Bonilla",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "ioi240036r13",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1038/s41579-022-00846-2",
"article-title": "Long COVID: major findings, mechanisms and recommendations.",
"author": "Davis",
"doi-asserted-by": "publisher",
"first-page": "133",
"issue": "3",
"journal-title": "Nat Rev Microbiol",
"key": "ioi240036r14",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.3389/fmicb.2021.698169",
"article-title": "Long COVID or Post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms.",
"author": "Proal",
"doi-asserted-by": "publisher",
"journal-title": "Front Microbiol",
"key": "ioi240036r15",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.7554/eLife.86014",
"article-title": "Immune mechanisms underlying COVID-19 pathology and post-acute sequelae of SARS-CoV-2 infection (PASC).",
"author": "Mohandas",
"doi-asserted-by": "publisher",
"journal-title": "Elife",
"key": "ioi240036r16",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.1038/s41590-023-01601-2",
"article-title": "SARS-CoV-2 reservoir in post-acute sequelae of COVID-19 (PASC).",
"author": "Proal",
"doi-asserted-by": "publisher",
"first-page": "1616",
"issue": "10",
"journal-title": "Nat Immunol",
"key": "ioi240036r17",
"volume": "24",
"year": "2023"
},
{
"DOI": "10.1017/ice.2020.1273",
"article-title": "Understanding viral shedding of severe acute respiratory coronavirus virus 2 (SARS-CoV-2): review of current literature.",
"author": "Fontana",
"doi-asserted-by": "publisher",
"first-page": "659",
"issue": "6",
"journal-title": "Infect Control Hosp Epidemiol",
"key": "ioi240036r18",
"volume": "42",
"year": "2021"
},
{
"DOI": "10.1056/NEJMc2031364",
"article-title": "Persistence and evolution of SARS-CoV-2 in an immunocompromised host.",
"author": "Choi",
"doi-asserted-by": "publisher",
"first-page": "2291",
"issue": "23",
"journal-title": "N Engl J Med",
"key": "ioi240036r19",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/S2666-5247(20)30172-5",
"article-title": "SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis.",
"author": "Cevik",
"doi-asserted-by": "publisher",
"first-page": "e13",
"issue": "1",
"journal-title": "Lancet Microbe",
"key": "ioi240036r20",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.1016/j.medj.2022.04.001",
"article-title": "Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection.",
"author": "Natarajan",
"doi-asserted-by": "publisher",
"first-page": "371",
"issue": "6",
"journal-title": "Med",
"key": "ioi240036r21",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1002/jmv.v95.1",
"article-title": "Liquid biomarkers of macrophage dysregulation and circulating spike protein illustrate the biological heterogeneity in patients with post-acute sequelae of COVID-19.",
"author": "Schultheiß",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "J Med Virol",
"key": "ioi240036r22",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac722",
"article-title": "Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae.",
"author": "Swank",
"doi-asserted-by": "publisher",
"first-page": "e487",
"issue": "3",
"journal-title": "Clin Infect Dis",
"key": "ioi240036r23",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1002/ana.v91.6",
"article-title": "SARS-CoV-2 and mitochondrial proteins in neural-derived exosomes of COVID-19.",
"author": "Peluso",
"doi-asserted-by": "publisher",
"first-page": "772",
"issue": "6",
"journal-title": "Ann Neurol",
"key": "ioi240036r24",
"volume": "91",
"year": "2022"
},
{
"DOI": "10.1016/j.mehy.2020.109907",
"article-title": "Periodontal pockets: a potential reservoir for SARS-CoV-2?",
"author": "Badran",
"doi-asserted-by": "publisher",
"journal-title": "Med Hypotheses",
"key": "ioi240036r25",
"volume": "143",
"year": "2020"
},
{
"DOI": "10.1053/j.gastro.2022.04.037",
"article-title": "Postacute COVID-19 is characterized by gut viral antigen persistence in inflammatory bowel diseases.",
"author": "Zollner",
"doi-asserted-by": "publisher",
"first-page": "495",
"issue": "2",
"journal-title": "Gastroenterology",
"key": "ioi240036r26",
"volume": "163",
"year": "2022"
},
{
"DOI": "10.1136/gutjnl-2021-324280",
"article-title": "Residual SARS-CoV-2 viral antigens detected in GI and hepatic tissues from five recovered patients with COVID-19.",
"author": "Cheung",
"doi-asserted-by": "publisher",
"first-page": "226",
"issue": "1",
"journal-title": "Gut",
"key": "ioi240036r27",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-05542-y",
"article-title": "SARS-CoV-2 infection and persistence in the human body and brain at autopsy.",
"author": "Stein",
"doi-asserted-by": "publisher",
"first-page": "758",
"issue": "7941",
"journal-title": "Nature",
"key": "ioi240036r28",
"volume": "612",
"year": "2022"
},
{
"DOI": "10.1016/j.ebiom.2021.103230",
"article-title": "SARS-CoV-2 persistence is associated with antigen-specific CD8 T-cell responses.",
"author": "Vibholm",
"doi-asserted-by": "publisher",
"journal-title": "EBioMedicine",
"key": "ioi240036r29",
"volume": "64",
"year": "2021"
},
{
"DOI": "10.1371/journal.ppat.1009037",
"article-title": "On the whereabouts of SARS-CoV-2 in the human body: a systematic review.",
"author": "Trypsteen",
"doi-asserted-by": "publisher",
"issue": "10",
"journal-title": "PLoS Pathog",
"key": "ioi240036r30",
"volume": "16",
"year": "2020"
},
{
"DOI": "10.5858/arpa.2021-0519-SA",
"article-title": "Late complications of COVID-19.",
"author": "Roden",
"doi-asserted-by": "publisher",
"first-page": "791",
"issue": "7",
"journal-title": "Arch Pathol Lab Med",
"key": "ioi240036r31",
"volume": "146",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2022.939989",
"article-title": "Case report: persistence of residual antigen and RNA of the SARS-CoV-2 virus in tissues of two patients with long COVID.",
"author": "Goh",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "ioi240036r32",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1001/jamainternmed.2023.0743",
"article-title": "Association of treatment with nirmatrelvir and the risk of post-COVID-19 condition.",
"author": "Xie",
"doi-asserted-by": "publisher",
"first-page": "554",
"issue": "6",
"journal-title": "JAMA Intern Med",
"key": "ioi240036r33",
"volume": "183",
"year": "2023"
},
{
"DOI": "10.1136/bmj-2022-074572",
"article-title": "Molnupiravir and risk of post-acute sequelae of COVID-19: cohort study.",
"author": "Xie",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "ioi240036r34",
"volume": "381",
"year": "2023"
},
{
"DOI": "10.1093/qjmed/hcab297",
"article-title": "Risk factors and incidence of Long-COVID syndrome in hospitalized patients: does remdesivir have a protective effect?",
"author": "Boglione",
"doi-asserted-by": "publisher",
"first-page": "865",
"issue": "12",
"journal-title": "QJM",
"key": "ioi240036r35",
"volume": "114",
"year": "2022"
},
{
"DOI": "10.3390/jcm12124158",
"article-title": "Treatment of COVID-19 during the acute phase in hospitalized patients decreases post-acute sequelae of COVID-19.",
"author": "Badenes Bonet",
"doi-asserted-by": "publisher",
"first-page": "4158",
"issue": "12",
"journal-title": "J Clin Med",
"key": "ioi240036r36",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.7326/M23-1394",
"article-title": "Effectiveness of nirmatrelvir-ritonavir against the development of post-COVID-19 conditions among US veterans: a target trial emulation.",
"author": "Ioannou",
"doi-asserted-by": "publisher",
"first-page": "1486",
"issue": "11",
"journal-title": "Ann Intern Med",
"key": "ioi240036r37",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.1038/s41467-022-33825-5",
"article-title": "Effect of remdesivir post hospitalization for COVID-19 infection from the randomized SOLIDARITY Finland trial.",
"author": "Nevalainen",
"doi-asserted-by": "publisher",
"first-page": "6152",
"issue": "1",
"journal-title": "Nat Commun",
"key": "ioi240036r38",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1002/jmv.v96.1",
"article-title": "Association of nirmatrelvir for acute SARS-CoV-2 infection with subsequent long COVID symptoms in an observational cohort study.",
"author": "Durstenfeld",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "J Med Virol",
"key": "ioi240036r39",
"volume": "96",
"year": "2024"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19.",
"author": "Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "ioi240036r40",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.14218/ERHM.2022.00045",
"article-title": "The use of nirmatrelvir-ritonavir in a case of breakthrough long COVID.",
"author": "Geng",
"doi-asserted-by": "publisher",
"first-page": "394",
"issue": "4",
"journal-title": "Exploratory Research and Hypothesis in Medicine",
"key": "ioi240036r41",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.20411/pai.v7i1",
"article-title": "Effect of oral nirmatrelvir on long COVID symptoms: 4 cases and rationale for systematic studies.",
"author": "Peluso",
"doi-asserted-by": "publisher",
"first-page": "95",
"issue": "1",
"journal-title": "Pathog Immun",
"key": "ioi240036r42",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1038/s41598-021-95565-8",
"article-title": "More than 50 long-term effects of COVID-19: a systematic review and meta-analysis.",
"author": "Lopez-Leon",
"doi-asserted-by": "publisher",
"first-page": "16144",
"issue": "1",
"journal-title": "Sci Rep",
"key": "ioi240036r44",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1038/s41591-022-01909-w",
"article-title": "Symptoms and risk factors for long COVID in non-hospitalized adults.",
"author": "Subramanian",
"doi-asserted-by": "publisher",
"first-page": "1706",
"issue": "8",
"journal-title": "Nat Med",
"key": "ioi240036r45",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.3389/fneur.2023.1090747",
"article-title": "Myalgic encephalomyelitis/chronic fatigue syndrome is common in post-acute sequelae of SARS-CoV-2 infection (PASC): results from a post-COVID-19 multidisciplinary clinic.",
"author": "Bonilla",
"doi-asserted-by": "publisher",
"journal-title": "Front Neurol",
"key": "ioi240036r46",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.3389/fneur.2022.1012668",
"article-title": "Characterization of autonomic symptom burden in long COVID: a global survey of 2,314 adults.",
"author": "Larsen",
"doi-asserted-by": "crossref",
"journal-title": "Front Neurol",
"key": "ioi240036r47",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1097/HCR.0000000000000336",
"article-title": "1-Minute Sit-to-Stand Test: systematic review of procedures, performance, and clinimetric properties.",
"author": "Bohannon",
"doi-asserted-by": "publisher",
"first-page": "2",
"issue": "1",
"journal-title": "J Cardiopulm Rehabil Prev",
"key": "ioi240036r49",
"volume": "39",
"year": "2019"
},
{
"DOI": "10.1093/biomet/72.2.359",
"article-title": "Combining dependent tests with incomplete repeated measurements.",
"author": "Wei",
"doi-asserted-by": "publisher",
"first-page": "359",
"issue": "2",
"journal-title": "Biometrika",
"key": "ioi240036r51",
"volume": "72",
"year": "1985"
},
{
"DOI": "10.1093/biostatistics/4.2.223",
"article-title": "Combining dependent tests for linkage or association across multiple phenotypic traits.",
"author": "Xu",
"doi-asserted-by": "publisher",
"first-page": "223",
"issue": "2",
"journal-title": "Biostatistics",
"key": "ioi240036r52",
"volume": "4",
"year": "2003"
},
{
"DOI": "10.1016/j.cell.2022.01.014",
"article-title": "Multiple early factors anticipate post-acute COVID-19 sequelae.",
"author": "Su",
"doi-asserted-by": "publisher",
"first-page": "881",
"issue": "5",
"journal-title": "Cell",
"key": "ioi240036r54",
"volume": "185",
"year": "2022"
},
{
"DOI": "10.1136/bmjmed-2022-000385",
"article-title": "Effect of covid-19 vaccination on long COVID: systematic review.",
"author": "Byambasuren",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "BMJ Med",
"key": "ioi240036r55",
"volume": "2",
"year": "2023"
},
{
"DOI": "10.1001/jamainternmed.2023.0750",
"article-title": "Risk factors associated with post-COVID-19 condition: a systematic review and meta-analysis.",
"author": "Tsampasian",
"doi-asserted-by": "publisher",
"first-page": "566",
"issue": "6",
"journal-title": "JAMA Intern Med",
"key": "ioi240036r56",
"volume": "183",
"year": "2023"
},
{
"DOI": "10.1038/s41467-022-29513-z",
"article-title": "Course of post COVID-19 disease symptoms over time in the COMPARE long COVID prospective e-cohort.",
"author": "Tran",
"doi-asserted-by": "publisher",
"first-page": "1812",
"issue": "1",
"journal-title": "Nat Commun",
"key": "ioi240036r57",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1136/bmjgh-2022-011276",
"article-title": "Conceptualising the episodic nature of disability among adults living with long COVID: a qualitative study.",
"author": "O’Brien",
"doi-asserted-by": "publisher",
"issue": "3",
"journal-title": "BMJ Glob Health",
"key": "ioi240036r58",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.1136/bmj-2022-074425",
"article-title": "Recovery and symptom trajectories up to two years after SARS-CoV-2 infection: population based, longitudinal cohort study.",
"author": "Ballouz",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "ioi240036r59",
"volume": "381",
"year": "2023"
},
{
"DOI": "10.1016/j.ijid.2023.05.007",
"article-title": "Trajectories of the evolution of post-COVID-19 condition, up to two years after symptoms onset.",
"author": "Servier",
"doi-asserted-by": "publisher",
"first-page": "67",
"journal-title": "Int J Infect Dis",
"key": "ioi240036r60",
"volume": "133",
"year": "2023"
},
{
"DOI": "10.1001/jama.2023.8823",
"article-title": "Development of a definition of postacute sequelae of SARS-CoV-2 infection.",
"author": "Thaweethai",
"doi-asserted-by": "publisher",
"first-page": "1934",
"issue": "22",
"journal-title": "JAMA",
"key": "ioi240036r61",
"volume": "329",
"year": "2023"
},
{
"DOI": "10.1136/bmj-2022-070230",
"article-title": "Development and validation of the symptom burden questionnaire for long COVID (SBQ-LC): rasch analysis.",
"author": "Hughes",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "ioi240036r62",
"volume": "377",
"year": "2022"
},
{
"DOI": "10.1038/s41573-019-0034-3",
"article-title": "Adaptive platform trials: definition, design, conduct and reporting considerations.",
"author": "Adaptive Platform Trials Coalition",
"doi-asserted-by": "publisher",
"first-page": "797",
"issue": "10",
"journal-title": "Nat Rev Drug Discov",
"key": "ioi240036r68",
"volume": "18",
"year": "2019"
},
{
"key": "ioi240036r3",
"unstructured": "US Centers for Disease Control and Prevention. Long COVID-19—Household Pulse Survey—COVID-19. Published October 11, 2023. Accessed October 18, 2023. https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm"
},
{
"key": "ioi240036r43",
"unstructured": "US Centers for Disease Control and Prevention. COVID-19 Vaccination: Clinical & Professional Resources. Published October 4, 2023. Accessed February 1, 2024. https://www.cdc.gov/vaccines/covid-19/index.html"
},
{
"key": "ioi240036r48",
"unstructured": "HealthMeasures. Patient-Reported Outcomes Measurement Information System. Accessed February 1, 2024. https://www.healthmeasures.net/explore-measurement-systems/promis"
},
{
"key": "ioi240036r50",
"unstructured": "eProvide Mapi Research Trust. Patient Global Impressions scale: Change, Improvement, Severity. Published October 5, 2023. Accessed February 1, 2024. https://eprovide.mapi-trust.org/instruments/patient-global-impressions-scale-change-improvement-severity"
},
{
"key": "ioi240036r53",
"unstructured": "The R Project for Statistical Computing. R software. Accessed October 31, 2023. https://www.r-project.org/"
},
{
"key": "ioi240036r63",
"unstructured": "Zimmerman? KO. RECOVER-VITAL: a platform protocol for evaluation of interventions for viral persistence, viral reactivation, and immune dysregulation in post-acute sequelae of SARS-CoV-2 infection (PASC). 2024. Accessed December 31, 2023. https://clinicaltrials.gov/study/NCT05595369"
},
{
"key": "ioi240036r64",
"unstructured": "Krumholz? HM. An Interventional decentralized phase 2, randomized, double-blind, 2-arm study to investigate the efficacy and safety of orally administered nirmatrelvir/ritonavir compared with placebo/ritonavir in participants with long COVID. 2023. Accessed December 31, 2023. https://clinicaltrials.gov/study/NCT05668091"
},
{
"key": "ioi240036r65",
"unstructured": "Brodin? P. An interventional, double-blinded, 2-arm study to investigate the efficacy of orally administered nirmatrelvir/ritonavir compared with placebo/ritonavir in non-hospitalized adult participants suffering from post-COVID. 2023. Accessed December 31, 2023. https://clinicaltrials.gov/study/NCT05823896"
},
{
"key": "ioi240036r66",
"unstructured": "Henrich? T. placebo-controlled, randomized trial of ensitrelvir (s-217622) for viral persistence and inflammation in people experiencing long COVID (PREVAIL-LC). 2023. Accessed December 31, 2023. https://clinicaltrials.gov/study/NCT06161688"
},
{
"key": "ioi240036r67",
"unstructured": "Peluso? M. An exploratory, randomized, double-blind placebo-controlled study to assess the safety of an anti-SARS-CoV-2 monoclonal antibody and response to treatment in individuals with long COVID (OutSMART-LC). 2024. Accessed December 31, 2023. https://clinicaltrials.gov/study/NCT05877508"
}
],
"reference-count": 68,
"references-count": 68,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2819901"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [
"The STOP-PASC Randomized Clinical Trial"
],
"title": "Nirmatrelvir-Ritonavir and Symptoms in Adults With Postacute Sequelae of SARS-CoV-2 Infection",
"type": "journal-article"
}